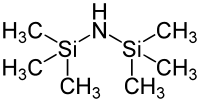
Hexamethyldisilazane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,1,1,3,3,3-hexamethyldisilazane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 19 NSi 2 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 161.39 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.78 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−82 ° C |
|||||||||||||||
| boiling point |
127 ° C |
|||||||||||||||
| Vapor pressure |
20 h Pa (20 ° C) |
|||||||||||||||
| solubility |
decomposes in water |
|||||||||||||||
| Refractive index |
1.4069-1.4089 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Hexamethyldisilazane , short HMDS , molecular formula C 6 H 19 NSi 2 , is widely used for the surface modification of solid surfaces, such. As for waterproofing of silica particles or to improve the adhesion of photoresists for semiconductor surfaces . In the semiconductor industry, the chemical vapor deposition (CVD) method allows HMDS to be applied easily.
In organic chemistry , hexamethyldisilazane is used, among other things, to introduce trimethylsilyl groups . In comparison to chlorotrimethylsilane , the handling is more comfortable, since hexamethyldisilazane is quite insensitive to water and humidity. In addition, there is no need to add an auxiliary base , since only ammonia occurs as a by-product in the silylation . For this, a higher reaction temperature usually has to be used, which can be a disadvantage with sensitive substrates.
Extraction and presentation
Hexamethyldisilazane can be obtained by reacting trimethylsilyl chloride with ammonia .
properties
Hexamethyldisilazane is a colorless to yellow colored liquid with an amine- like odor that is highly flammable. The compound has a flash point below 15 ° C. The explosion range is between 0.8% by volume as the lower explosion limit (LEL) and 25.9% by volume as the upper explosion limit (UEL). The ignition temperature is 325 ° C. The substance therefore falls into temperature class T2. Moisture causes slow hydrolysis with the formation of hexamethyldisiloxane and ammonia . Upon contact with water, the compound is first hydrolyzed in a violent reaction with evolution of heat and formation of ammonia to trimethylsilanol, which is unstable and condenses with elimination of water to form water-insoluble hexamethyldisiloxane (bis (trimethylsilyl) ether). The heat of hydrolysis is −144.4 kJ mol −1 .
Hazard assessment
In 2014, hexamethyldisilazane was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The causes for the uptake of hexamethyldisilazane were concerns about high (aggregated) tonnage and high risk characterization ratio (RCR). The re-evaluation took place from 2014 and was carried out by Spain . A final report was then published, but no changes were recommended.
Individual evidence
- ↑ a b c d e f g h i j Entry on 1,1,1,3,3,3-hexamethyldisilazane in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on hexamethyldisilazane at ChemBlink , accessed on February 25, 2011.
- ↑ Phillip Laube: HMDS. In: Lexicon - Semiconductor Technology from A to Z. Accessed April 19, 2011 .
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 711.
- ↑ Entry on 1,1,1,3,3,3-hexamethyldisilazane. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ↑ AEBeezer, CT Mortimer: Heat of formation and bond energies. Part XV. Chlorotrimethylsilane and Hexamethyldisilazane. In: J. Chem. Soc. A, 1966, pp. 514-516, doi : 10.1039 / J19660000514 .
- ^ European Chemicals Agency (ECHA): Substance Evaluation Report and Conclusion Document .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 1,1,1,3,3,3-hexamethyldisilazane , accessed on March 26, 2019.



![{\ displaystyle \ mathrm {2 \ (CH_ {3}) _ {3} SiCl + 3 \ NH_ {3} \ longrightarrow [(CH_ {3}) _ {3} Si] _ {2} NH + 2 \ NH_ {4} Cl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/09e35aea51a98318ddd1b54b2bfdbe5062f7ef86)