Modafinil
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| 1: 1 mixture of ( R ) -form (left) and ( S ) -form (right) |
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Modafinil | ||||||||||||
| other names | |||||||||||||
| Molecular formula | C 15 H 15 NO 2 S | ||||||||||||
| Brief description |
white to almost white, crystalline, polymorphic powder |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| Mechanism of action |
largely unknown |
||||||||||||
| properties | |||||||||||||
| Molar mass | 273.35 g · mol -1 | ||||||||||||
| density |
1.342 g cm −3 (enantiomer) |
||||||||||||
| Melting point | |||||||||||||
| solubility |
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Modafinil is a drug used to treat narcolepsy . It was co-developed with Adrafinil in France in the 1980s. The substances belong to a group of psychostimulating drugs that keep you awake and which differ significantly from amphetamines in terms of their molecular structure .
Modafinil is the major metabolite of Adrafinil ; Adrafinil is thus a prodrug of Modafinil. Not to be confused with this is Armodafinil , the ( R ) -enantiomer of the racemic Modafinil.
Modafinil was only sold by the former pharmaceutical company Cephalon until 2011 , but is now available as a generic .
Areas of application and prescribing practice
Modafinil is approved in Germany for the treatment of excessive sleepiness that occurs as part of narcolepsy.
In 2011, the European Medicines Agency (EMA) recommended approval restrictions after a safety assessment process, as the risk / benefit ratio of Modafinil is no longer considered to be favorable for the following diseases:
- Moderate to severe Obstructive Sleep Apnea Syndrome (OSAS) with excessive sleepiness despite adequate nCPAP therapy ,
- Moderate to severe chronic shiftworker syndrome with excessive sleepiness in patients on night shift change (SWSD) when other sleep hygiene measures have not led to any improvement.
In the US, however, Modafinil continues to be approved for the treatment of excessive sleepiness associated with narcolepsy, obstructive sleep apnea syndrome, and chronic shiftworker syndrome.
A prescription for other diseases is done off-label (e.g. chronic fatigue syndrome (CFS), ADHD or depression ) and is usually not reimbursed by the health insurance companies ( recourse liability of the doctors). In Germany, there are only a few amphetamine-like or stimulating drugs on the market and there is usually no intoxicating effect. Modafinil also has a significantly subliminal effect, especially in direct comparison to illegal stimulants such as cocaine or (meth) amphetamine, and therefore has a significantly lower addictive potential.
Contraindications and restrictions on use
Modafinil may not exceed a history known addictions not be applied. Simultaneous treatment with prazosin must not be used.
Modafinil should only be used with caution if the patient suffers from severe anxiety or has a history of psychotic illnesses. Modafinil should also only be used with particular caution in the case of severe liver or kidney diseases , high blood pressure and cardiovascular diseases .
A side effect is a dose-dependent increase in liver function indicators such as alkaline phosphatase and γ-glutamyltransferase (GGT) in the blood analysis, the latter also being typical for regular alcohol consumption.
Modafinil should be discontinued immediately after skin rashes occur, otherwise life-threatening hypersensitivity reactions may occur.
Based on case reports, it is suspected that the use of Modafinil during pregnancy can lead to severe, congenital malformations. A specific malformation pattern was not observed, as the manufacturer informed in a Rote-Hand-Brief in May 2019. Modafinil should therefore not be used during pregnancy, and patients of childbearing potential must use a reliable method of contraception.
pharmacology
Mode of action
The exact mechanism of action of Modafinil is not known. Part of the effect is caused by a centrally mediated, selective α 1 agonism. It is considered certain that the active ingredient increases the concentration of certain neurotransmitters such as B. dopamine , serotonin or norepinephrine increased.
Metabolism
Modafinil is rapidly absorbed, with maximum plasma concentrations being reached two to four hours after ingestion. The active ingredient has a moderate binding to plasma proteins (approx. 60%), especially to albumin . The plasma half-life is 10–12 hours with a single dose and 15 hours with continuous intake at steady state . Effective metabolites are not known.
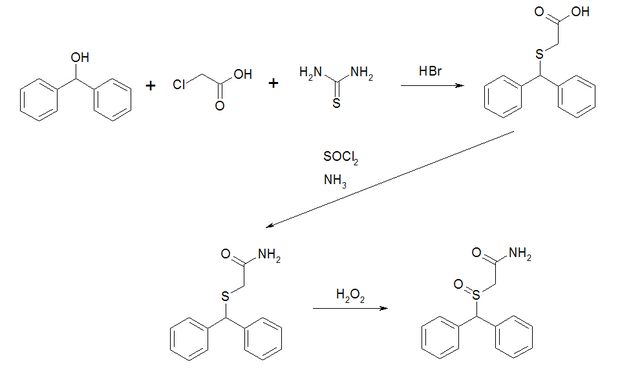
synthesis
The synthesis of racemic Modafinil starts from benzhydrol , which is reacted with chloroacetic acid and thiourea to form 2- (diphenylmethylthio) acetic acid. This is converted into the corresponding acid amide via the acid chloride. The subsequent oxidation with hydrogen peroxide gives the sulfoxide . The result is the racemate, a 1: 1 mixture of ( R ) -enantiomer and ( S ) -enantiomer of the drug.
The production of enantiomerically pure Modafinil starts from the racemic 2- (diphenylmethylsulfinyl) acetic acid, which is coupled with ( R ) -4-phenyl-thiazolidine-2-thione in the presence of DCC . The resulting pair of diastereomers can be separated chromatographically . Enantiomerically pure Modafinil is then obtained by reacting the pure diastereomers with ammonia water.
Legal status
In Germany, Modafinil was released from the narcotics regulations in March 2008 and made the normal prescription requirement (prescription requirement ).
In Switzerland, Modafinil is classified in dispensing category A, so a prescription entitles it to be purchased once at the pharmacy.
Modafinil requires a prescription in many other countries as well. In the USA, Modafinil is subject to a prescription requirement according to a graduated system that corresponds to the German narcotics prescription. It is classified there in the lowest category (Schedule) C IV.
Adrafinil- containing medicines, on the other hand, can be bought without a prescription in some countries. Adrafanil has the same effect as Modafinil, but the effect is delayed due to the prodrug effect .
abuse
Similar to methylphenidate , it is consumed before exams or work to improve performance. A placebo-controlled study found some positive effects on cognitive performance with a dose of 100–200 mg.
In the United States, sales of Provigil (American trade name of Modafinil) rose from $ 196 million in 2002 to $ 988 million in 2008. Studies have shown evidence of addiction. The long-term effects of Modafinil have not been studied; however, the US military recommends that its soldiers take Provigil before long stressful missions.
Modafinil is a prohibited doping substance in sports . In the meantime doping cases have become known. A prominent case is the US athlete and sprint world champion Kelli White .
Development and marketing
The manufacturer is Cephalon France (formerly Laboratoire L.Lafon). Merckle GmbH in Blaubeuren used to be the distributor. The German subsidiary of the American biopharmaceutical company Cephalon, Cephalon GmbH, Martinsried, has been selling the drug since 2002 .
Modafinil was licensed in the United States in 1993 by Frank Baldino , founder of the Cephalon company . In 2006, Cephalon had Modafinil alone in sales of $ 727 million. In February 2007, the company received an official warning from the US Food and Drug Administration ( FDA ) for illegally advertising the use of Modafinil in indications other than those approved. Cephalon was taken over by Teva .
After the protection periods had expired, various generics providers were added from 2011 , such as Glenmark Arzneimittel, Heumann Pharma, Aurobindo Pharma and Neuraxpharm Arzneimittel.
Trade names
Vigil (D), Modasomil (A, CH), Provigil (USA), Alertec (CAN), as well as a generic (A)
Web links
- Modafinil . In: Erowid . (English)
- Brain doping: eyes straight ahead . Telepolis
- Modafinil (PDF; 245 kB) FDA
- 11 steps to a better brain , NewScientist, May 28, 2005.
Individual evidence
- ↑ a b c d European Pharmacopoeia Commission (Ed.): European Pharmacopoeia 5th Edition . tape 5.0-5.7 , 2006.
- ^ Y. In, K. Tomoo, T. Ishida, Y. Sakamoto: Crystal and Molecular Structure of an (S) - (+) - Enantiomer of Modafinil, a Novel Wake-Promoting Agent . In: Chem. Pharm. Bull. 52, 2004, pp. 1186-1189.
- ↑ a b Entry on Modafinil. In: Römpp Online . Georg Thieme Verlag, accessed on September 30, 2014.
- ↑ a b A. Osorio-Lozada, T. Prisinzano, F. Olivo: Synthesis and determination of the absolute stereochemistry of the enantiomers of afrafinil and modafinil . In: Tetrahedron: Asymmetry , 15, 2004, pp. 3811-3815, doi: 10.1016 / j.tetasy.2004.10.019 .
- ↑ a b c d Physicians' Desk Reference . 62nd edition, Thomson Health Care Inc., Montvale NJ 2008, p. 988.
- ↑ Modafinil data sheet from Sigma-Aldrich , accessed on February 5, 2018 ( PDF ).
- ^ Norton W. Milgram et al. (1999): Adrafinil: A Novel Vigilance Promoting Agent. In: CNS Drug Reviews. 5, p. 193. doi : 10.1111 / j.1527-3458.1999.tb00100.x .
- ↑ Rote-Hand-Brief on Vigil (Modafinil): Restriction of the indication and important safety information for use , BfArM, February 7, 2011.
- ↑ technical information Provigil FDA (PDF; 245 KB).
- ↑ J. Biederman, SR Pliszka: Modafinil improves symptoms of attention-deficit / hyperactivity disorder across subtypes in children and adolescents. . In: J Pediatr . 152, No. 3, 2008, pp. 394-399. doi : 10.1016 / j.jpeds.2007.07.052 . PMID 18280848 .
- ↑ drugcom: modafinil. Retrieved April 10, 2020 .
- ↑ Product information on VIGIL® ( Memento from September 28, 2007 in the Internet Archive ) of the Yellow List .
- ↑ Vigil package insert.
- ↑ Life- threatening reactions to narcolepsy medication ( memento of the original dated February 5, 2018 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. . In: Deutsches Ärzteblatt, October 25, 2007.
- ↑ Possible risk of severe congenital malformations , Rote-Hand-Brief on Modafinil from May 9, 2019, accessed on May 10, 2019.
- ↑ Mutschler, Ernst: Mutschler drug effects. Pharmacology, clinical pharmacology, toxicology. 10th edition. Stuttgart. 2013.
- ↑ drugcom: modafinil. Retrieved April 14, 2020 .
- ↑ Specialist information Vigil, as of January 2011.
- ^ A. Kleemann , J. Engel, B. Kutscher: Pharmaceutical Substances . Thieme Medical Publishers 2001, ISBN 1-58890-031-2 .
- ↑ Twenty-first ordinance amending the regulations on narcotics (21st BtMÄndV).
- ↑ dak.de ( Memento from June 26, 2011 in the Internet Archive ) (PDF).
- ↑ DC Turner, TW Robbins, L. Clark, AR Aron, J. Dowson, BJ Sahakian: Cognitive enhancing effects of modafinil in healthy volunteers. In: Psychopharmacology. Vol. 165, number 3, January 2003, pp. 260-269, doi: 10.1007 / s00213-002-1250-8 . PMID 12417966 .
- ↑ Jörg Auf dem Hövel: Brain doping: eyes straight ahead . Telepolis , October 23, 2007.
- ↑ Warning Letter: Provigil® (modafinil) Tablets. (PDF; 6.3 MB) Food and Drug Administration, February 27, 2007.