Adrafinil
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
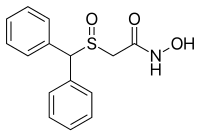
|
||||||||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Adrafinil | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 15 H 15 NO 3 S | |||||||||||||||||||||
| Brief description |
white solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 289.35 g · mol -1 | |||||||||||||||||||||
| solubility |
soluble in DMSO (35 mg / mL) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Adrafinil is a drug used to treat sleep disorders such as B. Narcolepsy . The substance belongs to a group of psychostimulating drugs that differ significantly in their molecular structure from the amphetamine- based stimulants .
Adrafinil is a prodrug that only becomes effective when it is metabolized to Modafinil . In its effect, it corresponds to Modafinil, but the effect is delayed due to the metabolism step. Like Modafinil, it is a prohibited doping substance .
Adrafinil was marketed in France from 1985 under the product name Olmifon ® . After the French Medicines Agency announced the manufacturer Cephalon in April 2011 that it was planning a reassessment of the risk-benefit ratio of Olmifon , Cephalon ceased sales.
Stereoisomerism
Adrafinil is a sulfoxide and has a stereocenter on the sulfur atom, so it is chiral . The drug is used as a racemate , i.e. from a 1: 1 mixture of the ( R ) form and the mirror image of the ( S ) form.
Prescription Status
In Germany, Adrafinil does not require a prescription . There are no approved finished medicinal products in Germany, Austria or Switzerland.
Individual evidence
- ↑ a b c d e Data sheet Adrafinil ≥ 98% (HPLC), solid from Sigma-Aldrich , accessed on March 20, 2011 ( PDF ).
- ↑ The 2009 Prohibited List WORLD ANTI-DOPING CODE Valid 1 January 2009 ( Memento from July 19, 2014 in the Internet Archive ) (PDF; 177 kB).
- ↑ Point d'information sur les dossiers discutés en commission d'AMM Séance , December 2, 2011.
- ↑ Appendix 1 of the Medicinal Prescription Ordinance (AMVV).