Amfepramon
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
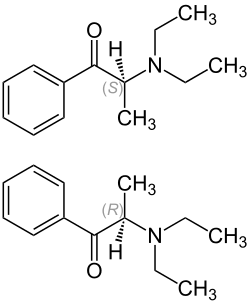
|
||||||||||||||||||||||
| 1: 1 mixture of ( R ) -form (top) and ( S ) -form (bottom) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Amfepramon | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 13 H 19 NO | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 205.30 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
168 ° C (hydrochloride, decomposition) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Amfepramon (also diethylpropion ) is a synthetic chemical compound from the group of phenethylamines . As an amphetamine derivative, it is one of the cathinones and its structure is similar to the antidepressant bupropion .
In Germany it is approved as a drug for the supportive treatment of overweight patients.
Mechanism of action
Amfepramon acts as an indirect alpha- sympathomimetic both systemically on the cardiovascular system and certain organs, as well as on brain activity in the central nervous system by crossing the blood-brain barrier . It can therefore be counted among the psychostimulants .
The active ingredient suppresses needs such as sleep, appetite, thirst and urination ( diuresis ). It increases for the duration of the effect the mental and physical performance, increases concentration, attention and alertness . In the body, the substance causes the indirect release of the catecholamines norepinephrine and dopamine , which are mainly responsible for the resulting effects. Blood pressure and heart rate increase, and the lungs are supplied with more blood.
In the brain, however, the substance blocks neither the dopamine transporters nor the transporters of norepinephrine. Instead, it acts as a prodrug to potent metabolites, mainly ethcathinone and diethylnorpseudoephedrine . Ethcathinone mainly causes a strong release of norepinephrine, which is why it is classified as a norepinephrine releasing agent (NRA). Further effective metabolites are created by reducing the keto group.
use
Amfepramone is rarely used in Germany for the short-term treatment of obesity. It requires a prescription and is available in the form of retarded and non-retarded capsules. It is therefore used therapeutically as an anorectic with a central nervous effect and is completely different from the more frequently prescribed orlistat in terms of its pharmacodynamics .
Like many low-potency phenethylamine derivatives, amfepramon together with other stimulants, including higher amounts of caffeine , has a not inconsiderable potential for abuse and dependence, which is why it was entered in Germany in Appendix 3 of the Narcotics Act . Preparations below a specified dose limit are exempt from special ordinance requirements under the Narcotics Prescription Ordinance.
Trade names
Regenon (D), Tenuate Retard (D)
Individual evidence
- ↑ Diethylpropion Hydrochloride, Material Safety Data Sheet (PDF). Spectrum Laboratory as of November 8, 2006.
- ↑ a b Datasheet Diethylpropion hydrochloride from Sigma-Aldrich , accessed on December 4, 2014 ( PDF ).
- ↑ a b c Entry on amfepramone in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Specialist information Regenon (Hormosan Pharma), as of August 2015.
- ^ Richard Rothman, Michael Baumann: Therapeutic Potential of Monoamine Transporter Substrates . In: Current Topics in Medicinal Chemistry . tape 6 , no. 17 , p. 1845-1859 , doi : 10.2174 / 156802606778249766 ( eurekaselect.com [accessed December 21, 2016]).
- ↑ Appendix III Marketable and prescribable narcotics (delivery according to BtMVV ).