Fosamprenavir
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
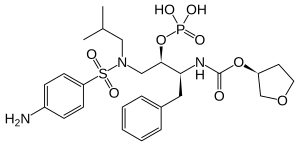
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Fosamprenavir | |||||||||||||||
| other names |
GW433908 |
|||||||||||||||
| Molecular formula | C 25 H 36 N 3 O 9 PS | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action |
Inhibition of HIV proteases |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 585.61 g mol −1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Fosamprenavir (fAPV, trade name: Telzir ® , manufacturer: GlaxoSmithKline ) is a drug that is used in oral dosage forms for the treatment of HIV infections and AIDS . It belongs to the group of HIV protease inhibitors that are usually combined with other antiviral drugs. See HAART , NNRTI , NRTI .
Fosamprenavir-containing tablets and suspensions were developed by Glaxo Wellcome and approved by the European Commission in July 2004 for the treatment of previously treated patients with HIV infection in the EU.
Indication and effectiveness
The medicine is approved for HIV infection in adults. Fosamprenavir itself is not effective; only the metabolite amprenavir , which is itself available as a medicinal substance, develops the antiviral effect. As a rule, fosamprenavir is boosted with ritonavir , which means that a second HIV protease inhibitor in a low dose inhibits the breakdown of fosamprenavir so that a higher active ingredient content remains in the body.
Mechanism of action, pharmacokinetics
Fosamprenavir is a further development of the HIV protease inhibitor amprenavir. By esterification with phosphoric acid a was prepared from amprenavir prodrug with diminished lipophilicity , the better absorption from the gastrointestinal tract allows. Fosamprenavir is hydrolyzed to amprenavir during absorption. This is almost completely bound to plasma proteins in the blood. The effect is to inhibit the viral HIV protease , which means that infectious viruses can no longer be formed in the host cell . Degradation and excretion takes place via the liver and stool, with the cytochrome P450 enzyme system playing the decisive role.
dosage
The recommended dosage for fosamprenavir is 700 mg twice daily (= 1 film-coated tablet ) together with 100 mg ritonavir twice daily. This means a significant reduction in the number of tablets compared to therapy with amprenavir.
Side effects, contraindications
Headache, dizziness, tiredness, nausea, vomiting, diarrhea and exanthema are common . Under certain circumstances, these can lead to life-threatening complications and discontinuation of therapy. The breakdown mechanism via the liver results in numerous interactions with other drugs. The combination of fosamprenavir / ritonavir, both inhibitors of the cytochrome CYP3A4 isoenzyme, aggravates this situation significantly. Treatments with additional medication therefore require careful monitoring.
literature
- JM Gatell: From amprenavir to GW433908 , J HIV Ther., 2001; 6 (4): 95-99.
Web links
- Drug Information (Engl.)
- MEROPS : Page on Fosamprenavir (engl.)
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.