Phenacetin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
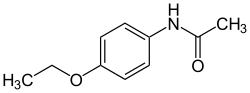
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Phenacetin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 10 H 13 NO 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 179.22 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.359 g cm −3 |
|||||||||||||||||||||
| Melting point |
134-135 ° C |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Phenacetin is an aminophenol - derivative and was used as drug for the treatment of pain and fever reduction used.
history
Phenacetin was made by the paint factories in 1888 . Friedr. Bayer & Co. in Elberfeld was the company's first drug. The raw material p -nitrophenol was produced as a by-product that was previously unusable in large quantities during the dye production of Benzoazurin G. The third step of acetylation was based on the competitor product Antifebrin from Kalle . The trademark registration “Phenacetin” was refused because other companies like the pharmaceutical factory JD Riedel OHG or Höchst Farbwerke also produced this active ingredient and sold it under the name Phenacetin.
properties
Phenacetin is a colorless and odorless white solid, hardly soluble in water and melts at around 135 ° C.
use
Phenacetin was used in numerous preparations against migraines , neuralgia and rheumatism . It became abusive because of its slightly euphoric effect - especially in combination with caffeine - to improve the performance of employees, e.g. B. in the watch industry, taken. Because of its health-damaging, especially kidney-damaging effects in combination with other pain medication ( phenacetin kidney or analgesic nephropathy , urothelial carcinoma ), this drug is no longer on the market as a finished drug (since 1986 in the Federal Republic of Germany, since 1990 in the GDR). In Germany and other western countries, pharmaceutical preparations (formulations) with phenacetin are considered questionable. Their production and distribution is therefore prohibited. Phenacetin has been replaced by the metabolite paracetamol . Phenacetin is also used as an extender for cocaine .
Individual evidence
- ↑ H. Wollmann, B. Skaletzki, A. Schaaf: [Determination of polarity of drugs. 14. Contributions to problems of use of plastic containers for liquid drug preparations]. In: The Pharmacy. Volume 29, Numbers 10-11, 1974 Oct-Nov, pp 708-711, PMID 4438412 .
- ↑ a b c d Entry on phenacetin in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ^ O'Neil, MJ (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1292
- ↑ Seidell A; Solubilities of Organic Compounds. NY, NY: d. Van Norstrand Co., Inc. (1941)
- ↑ Lide, DR CRC Handbook of Chemistry and Physics 86TH Edition 2005-2006. CRC Press, Taylor & Francis, Boca Raton, FL 2005, p. 3-236
- ↑ a b A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications , 4th edition (2001) Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 .
- ↑ milestones. 125 Years of Bayer ( Memento from October 19, 2013 in the Internet Archive ), pp. 90–93.
- ↑ Rejection of the brand name "Phenacetin" by the DPMA Munich 1888 ( Memento of December 2, 2013 in the Internet Archive ) (PDF; 660 kB). - The trade name "Phenacetin-Bayer" was therefore used subsequently, see Pharmazeutische Zeitung 1888, page 341f. - January 1896 new trademark registration "Phenacetin" by Bayer, see Pharmazeutische Zeitung 1896, pages 307 and 868.
- ↑ Medicines Commission of German Pharmacists: Questionable Prescription Medicines (PDF; 423 kB).
- ↑ BGH confirms the classification as a questionable drug according to § 5 AMG ( memo of April 10, 2013 in the Internet Archive ), accessed on May 9, 2012.


