Optical coherence tomography
Optical coherence tomography ( English optical coherence tomography , OCT ) is to obtain micron-resolution imaging technique for 2- and 3-dimensional images from scattering materials (e.g., biological tissue).
For this purpose, broadband light with a short coherence length is divided into two parts in a beam splitter. A part is directed to the sample. The other part runs through a reference section. The light reflected from the sample is superimposed with the reference light in an interferometer and thus brought into interference. Different structures along the optical axis (depth) can then be distinguished from the interference signal. Lateral scanning over the sample gives three-dimensional images.
OCT is analogous to ultrasound imaging ( sonography ), only that it uses light instead of sound.
The main area of application for OCT is medicine, primarily ophthalmology. Here, infrared light in the wavelength range from approx. 800 to 1400 nm is used as a rule (hence the term optical tomography ).
The strengths of OCT lie in the relatively high penetration depth (1–3 mm, depending on the wavelengths used) in scattering tissue with a high axial resolution (0.5–15 µm). The bandwidth of the light source used determines the axial resolution. Example: a superluminescent diode as a light source with a central wavelength of 1325 nm and a bandwidth of 97 nm has a coherence length of approx. 16 micrometers. The OCT resolution in the axial direction is then equal to half the coherence length, in the example 8 micrometers.
In order to obtain a complete 3D image, depending on the OCT measurement method, scans must be made over the object. If, for example, a volume of 1 mm × 1 mm × 1 mm is to be scanned with a resolution of 10 micrometers in all directions, this would require 1 million measurements in the worst case. However, depending on the OCT measurement method used, the axial measurement can be carried out simultaneously over the entire depth, for example. Then only 10,000 scans, corresponding to 10,000 measurements, are needed to measure a 1 mm × 1 mm × 1 mm volume with a resolution of 10 micrometers. Commercial OCT instruments (as of 2019) achieve scan rates of 85 kHz.
principle
“The principle of OCT is based on the technology of white light interferometry, which uses radiation with little temporal coherence and can be represented on the technical structure of a Michelson interferometer ”.
A light source (for example a superluminescent diode) illuminates the sample to be examined in the measuring arm (sample beam) of the white light interferometer via a beam splitter . The light transmitted by the beam splitter (reference beam) falls on a mirror in the reference arm and is reflected back by this. The sample beam and the reference beam meet again and interfere precisely when the difference between the paths covered by the two beams is less than the coherence length. The interference signal is recorded with a detector and then evaluated. By moving the mirror in the reference arm, interference signals are obtained from different depths of the sample if there are reflective structures there. Moving the mirror in the reference arm while measuring the interference signal at the same time allows the sample to be scanned axially. Since path length differences over the speed of light can also be specified as transit time differences, this OCT method is referred to as time domain (TD) OCT. In order to obtain a 3-dimensional image of the sample, the sample beam is moved laterally over the sample (scanned). The smallest possible lateral resolution in this case corresponds approximately to the diameter of the light beam. The axial depth resolution, on the other hand, is determined by the coherence length of the light used.
application

Areas of application are primarily in medicine : OCT is used primarily in ophthalmology as well as for early cancer diagnosis and skin examinations . Here, reflections at the interfaces of materials with different refractive indices are measured and a three-dimensional image is reconstructed. Such a reconstruction is called tomography .
OCT is currently used to examine the fundus or the posterior segment of the eye , as competing techniques such as For example, the confocal microscope cannot adequately image the fine layer structure of the approx. 250-300 µm thick retina due to the small pupil size and the large distance from the cornea to the retina. OCT is also used to measure the length of the eye during preliminary examinations for cataract operations . This is an important parameter for calculating the intraocular lens to be used . Other methods, on the other hand, are not suitable due to the high stress they place on the human eye or are impaired too much by the vitreous humor of the eye (e.g. high-resolution ultrasound ). In addition, contactless measurement is advantageous because the risk of infection and psychological stress are largely reduced.
The OCT examination of the retina is used to diagnose diseases of the retina such as macular degeneration (disease of the central retina), diabetic macular edema (fluid accumulation in the central retina in diabetic retinopathy ) and macular edema in retinal vein thrombosis ( central vein thrombosis, branch vein thrombosis). Furthermore, the investigation of individual retinal layers such as the retinal nerve fiber layer (RNFL) and the ganglion cell complex (GCC) are used to diagnose diseases of the optic nerve such as glaucoma or optic atrophy . Because of its importance, the OCT examination is a specialist standard in ophthalmology (treatment standard of an average specialist in ophthalmology). However, the OCT devices used in ophthalmological practices in Germany are quite different.
Another development of OCT examinations in the field of ophthalmology is the use of OCT angiography . In order to map the blood flow using OCT angiography, each B-scan of a volume scan is first repeated several times in quick succession at exactly the same position, and the temporal contrast differences at this position are analyzed. The comparative evaluation of all B-scans of a volume scan results in areas with constant contrast as well as areas with temporal contrast differences. These represent a blood flow so that the vascular system can be represented three-dimensionally within the area covered by the volume scan using OCT angiography. By segmenting between certain retinal layers, partial en-face representations of the microvasculature of these retinal areas can also be created at any depth.
Cardiovascular imaging is a new field of application for OCT . , The intravascular optical coherence tomography is a new, on infrared light based technique, the arteries may represent microns with a resolution of 10-20. Various preclinical and clinical series have shown that OCT enables reliable identification of intramural and luminal morphologies, e.g. B. plaques, thrombi , dissections as well as information about lumen and stent dimensions . Studies comparing IVUS and OCT have shown that OCT provides additional morphological information that allows improved plaque characterization.
OCT has a very large and growing application potential in the field of non-destructive testing . Some groups around the world are working on establishing OCT for quality control of products and processes in industry. There is a wide range of applications, particularly in the plastics industry (e.g. inline monitoring of extrusion processes , quality control of composite materials, etc.). Another area of application with great future potential (due to the high number of items / throughputs) is the monitoring of tablet coating processes in the pharmaceutical industry.
Axial resolution and bandwidth
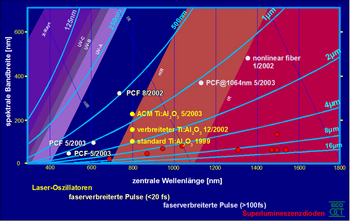
After initial experiments with light sources of limited bandwidth (a few nanometers), relatively broadband light sources with high spatial coherence became available and used. The systems were mostly based on superluminescence diodes with a bandwidth of several tens of nanometers (typ. 30 nm, corresponds to at least 30 µm resolution). In 1997 this resolution was increased tenfold (> 100 nm, corresponds to at least 3 µm axial resolution). The tomograms are thus very close to the histological sections (1 µm section thickness).
The following formula (derived from the Fourier ratio between the correlation width and the spectral width, measured at full width at half height ) enables the associated axial resolution to be calculated for a spectrum with a Gaussian distribution :
- = axial resolution
- = central wavelength
- = full spectral bandwidth at half the height of the spectrum (FWHM) Assumption: Gaussian spectrum
The dispersion in human tissue and especially in the vitreous humor of the eye destroys the coherence of the two arms. Skilful balancing of the dispersion in both arms, however, enables the coherence to be restored. The precision of ultra-high-resolution OCT has led to a rethink in ophthalmology, as ophthalmologists can suddenly get information they only knew from textbooks. This makes it possible to detect even the smallest changes in the early stages, which was difficult or impossible with other methods.
The latest developments in non-linear optics make it possible to develop light sources for other wavelength regions and with an even greater bandwidth (see picture).
sampling rate
In the time domain, the interference signal is sampled at any small intervals . However, the sampling rate has no influence on the resolution. The curve is therefore measured more precisely, but the smallest width of an individual signal does not become narrower. However , if the sampling rate falls below twice the carrier frequency of the signal, aliasing artifacts occur according to the Nyquist-Shannon sampling theorem .
Measurement methods
Because the autocorrelation (cross-correlation of a temporal signal with itself) is linked with the frequency spectrum of a function via the Fourier transformation , the analogous relationship between the optical spectrum and the interference signal applies in the optical domain. This is why one speaks on the one hand of the signal in the time domain (English time domain , TD) and on the other hand of the signal in the frequency domain (English frequency domain , FD). Put simply, this means that you either change the length of the reference arm and continuously measure the intensity of the interference without taking the spectrum into account ( time domain ), or record the interference of the individual spectral components ( frequency domain ). A variant of the FD-OCT records the individual spectral components one after the other by tuning the wavelength of the radiation source ( swept source , SS). SS-OCT therefore manages without a spectrometer for detection, but requires a radiation source that can be tuned in terms of wavelength. FD-OCT was only made possible by the availability of fast, sensitive cameras and fast computers.
The advantage of the FD method lies in the simple and fast simultaneous measurement. Here the complete information about the depth can be acquired simultaneously without the need for a mechanically movable part. This increases stability and speed. The difference between the two methods can also be seen in the fact that TD-OCT has to absorb the total power of the reference and measuring arm at each measuring point, but the interference component only makes up an extremely small part, whereby the noise of the total signal outweighs the useful component. When recording in the frequency domain (FD-OCT), only the corresponding spectral power is measured as the background in each spectral channel. This means that all interference from the other spectral ranges is lost. The required dynamics of the detector decrease with the total output per channel. Consequently, with the same sensitivity (= sensitivity for measuring the smallest reflectivities ), frequency range measurements require only a fraction of the radiated power. FD-OCT is far more effective than TD-OCT. This can be an important aspect for applications on the eye. In principle, the analogue to SS-OCT is also in the time domain, i. H. simultaneous measurement is possible, but it requires non-linear processes that only work with relatively high light intensities. However, this contradicts the highly sensitive measurement with measurement signal powers below the nanowatt range.
The Fourier transformation , however, works in complex number space , so both methods are only equivalent if the complex-valued functions are known. The final measurement signal should, however, show the time course of the reflectivity (= absolute value of the intensity in time ). reproduce, which is why there is ambiguity in intensity recordings in the frequency domain and the lack of complex-valued information. The result is the "flipping over of the image" with the conventional FD process. However, since the imaginary part of a function corresponds to a phase jump by 90 °, the complex-valued function can be produced by additional measurement with a reference arm shifted by 90 ° in the transit time (i.e. a quarter of the wavelength) and thus the complete time function can be reconstructed.
Sampling rate, line width and measurement depth
The sampling rate in the frequency domain is linked to the measurement depth via the Fourier transformation. A higher sampling rate or number of pixels of a detector within the same spectral range increases the range in which several objects can be clearly distinguished from one another. The same restriction applies here as in the time domain: If the line width, i.e. the smallest possible individual spectral line, is undershot, there is no longer any additional information when oversampling. (The line width is limited either by the light source for temporal encoding or by the imaging geometry and scattering effects in the spectrometer for spatial encoding ). After the Fourier transformation, a line width greater than the scanning density leads to a drop in the object intensity towards the edge of the spatial area. In the case of undersampling, multiple images also form outside the zero order of the local area, that is to say the area in the center of which the measuring arm and the reference arm are of equal length. When subsampling, objects outside the measuring range are reflected in.
OCT measurement methods
Many different methods for signal acquisition have been developed recently - the following is a systematic overview of all possible methods. The holographic processes are the spatial, transversal counterpart to the longitudinal, temporal frequency range of the optical transit time. There is therefore a Fourier relationship between the longitudinal transit time and the temporal frequency as well as between the transverse deflection and the transverse spatial frequency . In principle, a distinction is made between two subgroups, in which, on the one hand, the signal is time encoded , i.e. recorded sequentially, or spatially encoded, i.e. spatially split up but recorded simultaneously. Unsystematic terms such as “Fourier Domain OCT” or “Spectral OCT” are often used, but they are mostly confusing (confusion with spectroscopic OCT ) and imprecise (the frequency is correlated with time, not the wavelength) or sometimes meaningless (it does exist no Fourier domain). Nevertheless, they are given in the table below as alternative names for orientation .
| Time Domain (TD) | Frequency Domain (FD) | ||||
|---|---|---|---|---|---|
| Deep scan | sequential | simultaneously | sequential | simultaneously | |
| effort | mechanically high | electronically + optically high | optical + post processing high | optical + post processing high | |
| Light source | broadband | broadband | variable wavelength | broadband | |
| Interferometer | Beam splitter | expanded measuring beam | Beam splitter | Beam splitter | |
| scanner | movable reference arm mirror | static | static | static | |
| detector | simple, highly sensitive (diode) | Field (diodes, CCD or CMOS line-array) | simple, highly sensitive (diode) | complex, prism or grid + field | |
| 1D OCT | |||||
| systematic designation | 1D-teTD OCT | 1D-seTD OCT | 1D-teFD OCT | 1D-seFD OCT | |
| alternative name | scanning TD OCT | - | swept source OCT, spectral domain OCT |
Frequency Domain OCT, Fourier (Transform) OCT, Spectral Domain OCT |
|
| 2D OCT | |||||
| systematic designation | 2D-teTD OCT | 2D seTD OCT | 2D-teFD OCT | 2D seFD OCT | |
| alternative name | - | - | - | parallel spectral domain OCT | |
| Parallelizability | easy | medium | easy | heavy | |
| 2D orientation | en-face ( normal to the beam ) |
Cross section ( one axis in the direction of the beam) |
en-face | cross-section | |
| 3D-OCT | |||||
| systematic designation | 3D-teTD OCT | 3D seTD OCT | 3D teFD OCT | 3D-seFD OCT | |
| alternative name | en-face OCT, full field / frame OCT | - | time encoded frequency domain OCT | - | |
| Parallelizability | easy | - | easy | extremely complex | |
| Holographic illustration | |||||
| systematic designation | holo-teTD-OCT | - | holo-teFD-OCT | - | |
| alternative name | holographic OCT | - | holographic teFD OCT | - | |
The methods differ in their image quality and applicability, due to the use of different components. The FD methods in particular have the advantage of not wasting light and are much more sensitive. The goal is a high sensitivity with the use of as few movable components as possible and thus a high speed, for example 3D-teFD and holographic processes. On the other hand, the phase coherence is better with the potentially slower procedures. In addition, the alignment of the grid method and its grid density are important; In layered biological tissues, for example, a high grid density in the depth cross-section is usually desired, which is difficult to achieve with the fast, simple on-face methods.
Extensions
In addition to the purely topographical information, further data can be evaluated from the original signal. Thus, by measuring several successive tomograms at the same point, the local Doppler shift can be used to measure the speed (Doppler OCT). In addition, various material properties such as scattering, absorption, change in polarization ( polarization sensitive OCT ) and dispersion can be determined and displayed. In addition, attempts are made to mark tissue or to search only selectively for certain molecules (English molecular contrast OCT ).
advantages
The great technological advantage of OCT is the decoupling of the depth resolution from the transversal resolution. The measurement, which is based purely on optical reflection and therefore non-contact, eliminates the need for thin sections used in microscopy, which means that the method allows microscopic images in living tissue ( in vivo ).
Due to the high selectivity of the operating principle, very small signals (below nanowatts) can be detected and assigned to a certain depth, with low input power. This method is therefore also suitable for examining light-sensitive tissue.
The use of OCT is limited by the wavelength-dependent penetration depth of electromagnetic radiation into the examination subject and by the bandwidth-dependent resolution. Since 1996, highly developed broadband lasers have enabled the development of UHR-OCT ( ultra-high resolution OCT ), which has advanced depth resolution from several micrometers to fractions of micrometers. Subcellular structures in human cancer cells can be visualized in this way.
Similar procedures
OCT is related to other interferometric profiling processes (which can only measure surfaces) such as holography and optical coherence radar, which is used for the high-precision three-dimensional representation of surfaces in aircraft construction and the automotive industry .
In addition, the digital holography overlaps with the area of the OCT. Here the physical image is recorded in the Fourier plane and the interference pattern is extended to the entire volume using mathematical back-calculation. The advantage here is the independence from the focusing (which is numerically compensated), which only causes a drop in intensity, but no blurring. Numerical holography has the disadvantage that it is very sensitive to speckle and multiply scattered photons, which occur more frequently with scattering materials. In addition, like the “full-field” OCT variants, holography cannot benefit from the confocal advantage of suppressing crosstalk. There is also overlap in the phase modulation methods, in which the phase in the interference arm is primarily modulated. An alternative to OCT in medicine is multiphoton tomography , which enables higher resolutions, but the signal depth is limited to several hundred micrometers.
outlook
OCT is a relatively young procedure (first developed in the late 1980s) and is currently beginning to establish itself in various areas. Not all technical possibilities have been exhausted either. The low load on the examination subject, the high resolution and increasing speed make the method very attractive. New light sources, detectors and scanners will make it possible in the future to perform high-resolution three-dimensional microscopy on living tissue at video speed. The amount of data for such high quality recordings would reach a few gigavoxels per second; Current high-resolution OCT methods reach up to 250 megavoxels per second, although the level in 2000 was still below 100 kilovoxels per second. Ultra-high-speed OCT with lower sensitivity already achieves up to 60 gigavoxels per second through parallel detection.
Individual evidence
- ↑ SPECTRALIS OCT2 module. In: Manufacturer website for the SPECTRALIS OCT device. Heidelberg Engineering GmbH, accessed on November 5, 2019 (German).
- ↑ Cyriak Nathanael Schulz-Wackerbarth: Evaluation of the slit lamps Spectral Radar Optical Coherence Tomography (SL SR OCT) and comparison with SL OCT and Stratus OCT in physiological and pathological findings of the anterior and posterior segment. Dissertation, Lübeck 2011, https://www.zhb.uni-luebeck.de/epubs/ediss1019.pdf .
- ↑ Quality assurance of optical coherence tomography for diagnosing the fundus. (PDF) Retrieved January 28, 2019 .
- ^ GE Lang, C. Enders, JU Werner: [New Possibilities in Retinal Diagnostics Using OCT Angiography] . In: Clinical monthly sheets for ophthalmology . tape 233 , no. 5 , May 2016, ISSN 1439-3999 , p. 613-621 , doi : 10.1055 / s-0042-105325 , PMID 27187882 .
- ↑ Optical coherence tomography (OCT), Medical Clinic for Cardiology (CBF) at the Charité. Retrieved November 22, 2018 .
- ↑ OCT - Intracoronary Imaging, University of Munich. Retrieved November 22, 2018 .
- ↑ D. Markl et al .: Optical coherence tomography as a novel tool for in-line monitoring of a pharmaceutical film-coating process. In: European Journal of Pharmaceutical Sciences. , 55, 2014, pp. 58-67, doi: 10.1016 / j.ejps.2014.01.011 .
- ↑ Bin Liu, Mark E. Brezinski: Theoretical and practical considerations on detection performance of time domain, Fourier domain, and swept source optical coherence tomography . In: Journal of Biomedical Optics . tape 12 , 2007, ISSN 1083-3668 , p. 044007 , doi : 10.1117 / 1.2753410 .
- ↑ Wolfgang Drexler et al: Ultrahigh-resolution ophthalmic optical coherence tomography . In: Nature Medicine . tape 7 , no. 4 , 2001, p. 502-507 , doi : 10.1038 / 86589 ( Erratum. In: Nature Medicine. Volume 7, No. 5, 2001, p. 636).