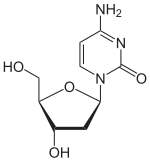
Decitabine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Decitabine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 12 N 4 O 4 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action |
DNA methyltransferase - inhibitor |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 228.21 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
200 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Decitabine (trade name: Dacogen ® , manufacturer: Janssen-Cilag ) is a synthetic nucleoside . It consists of β- D -deoxyribose (sugar) and a substituted triazine . It is a chemical analog of the nucleoside deoxycytidine . It is a cytostatic agent that acts as a DNA methyltransferase inhibitor (demethylating substance).
properties
The 5- aza- 2'-deoxycytidine differs chemically from deoxycytidine by a in the 5-position CH unit is formally replaced by a nitrogen atom. The pyrimidine backbone thus becomes a 1,3,5-triazine backbone. The analog with ribose is azacitidine .
|

|

|
| Deoxycytidine, dC | Decitabine | 5-azacytidine, 5-azaC |
effect
Decitabine was approved by the US FDA in 2006 as an orphan drug for the therapy of myelodysplastic syndromes (MDS). On September 20, 2012, the European Commission granted EU-wide approval for the treatment of adults over 65 years of age with acute myeloid leukemia (AML) . Decitabine has the same mechanism of action as 5-azacytidine . In a nationwide clinical study with over 220 patients, which was carried out between 2003 and 2009, the use of low-dose decitabine for the treatment of older AML patients was investigated. The aim of another study is how decitabine, in combination with valproic acid or the vitamin A preparation retinoic acid, works with regard to complete or at least partial remission and the survival of patients.
Early benefit assessment
Since 2011, newly approved drugs with new active ingredients have to undergo an " early benefit assessment " by the Federal Joint Committee (G-BA) based on Section 35a SGB V ( AMNOG ) if the pharmaceutical manufacturer wants to achieve a higher sales price than just the fixed amount . Only if there is an additional benefit can the drug manufacturer negotiate a price with the umbrella association of statutory health insurers (GKV Spitzenverband) . This also applies to decitabine. An initial assessment by the Institute for Quality and Efficiency in Health Care (IQWiG) was carried out in advance. The G-BA passed a resolution on the “additional benefit” of decitabine at the end of April 2013. According to the G-BA, a minor additional benefit was found.
Web links
- Entry for Decitabine in the Human Metabolome Database (HMDB) , accessed November 15, 2013.
- Label from Dacogen (PDF; 235 kB) FDA.
- Norbert Gattermann: Epigenetic treatment with 5-azacytidine and decitabine . German MDS Forum - Duisburg 2008.
- Registration entry. In: Community Register of medicinal products of the European Commission; Retrieved March 29, 2013.
Individual evidence
- ↑ a b c d Data sheet 5-Aza-2′-deoxycytidine from Sigma-Aldrich , accessed on November 5, 2016 ( PDF ).
- ↑ Entry on decitabine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Summary of the EPAR for the public . (PDF; 59 kB) European Medicines Agency.
- ↑ Implementation decision of the Commission of September 20, 2012 on the marketing authorization for the human medicinal product for orphan diseases "Dacogen - Decitabine" according to Regulation (EC) No. 726/2004 of the European Parliament and of the Council (PDF; 14.4 kB) European Commission.
- ^ Benjamin Waschow: Hope for elderly patients with acute myeloid leukemia. Freiburg University Medical Center, press release from April 25, 2012 from Informationsdienst Wissenschaft (idw-online.de), accessed on August 24, 2015.
- ↑ Decitabin - evaluation according to § 35a Abs. 1 Satz 10 SGB V (PDF; 123 kB) WebSite of the IQWiG, January 16, 2013; Retrieved May 2, 2013.
- ↑ G-BA makes three further decisions on new active ingredients. Press release G-BA, May 2, 2013.