Triazines
| Triazines | |||||||
| Surname | 1,2,3-triazine | 1,2,4-triazine | 1,3,5-triazine | ||||
| other names |
v -triazine vic -triazine |
as- triazine asym -triazine |
s -triazine sym -triazine |
||||
| Structural formula |  |
 |
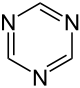
|
||||
| CAS number | 289-96-3 | 290-38-0 | 290-87-9 | ||||
| EC number | 808-989-8 | 206-028-1 | |||||
| ECHA info card | 100.236.897 | 100.005.481 | |||||
| PubChem | 123047 | 67520 | 9262 | ||||
| Wikidata | Q27117364 | Q27117363 | Q751744 | ||||
| Molecular formula | C 3 H 3 N 3 | ||||||
| Molar mass | 81.08 g mol −1 | ||||||
| Physical state | firmly | liquid | firmly | ||||
| Brief description | pale yellow oil | colorless, highly refractive, rhombohedral crystals |
|||||
| Melting point | 70 ° C | 17 ° C | 86 ° C | ||||
| boiling point | 156 ° C | 114 ° C | |||||
| density | 1.448 g cm −3 | 1.38 g cm −3 | |||||
| solubility | soluble in ethanol , diethyl ether ; Decomposes in water |
||||||
|
GHS labeling |
Caution |
|
danger |
||||
| H and P phrases | 302-315-319-335 | see above | 302-315-318-335 | ||||
| ? | see above | 261-280-305 + 351 + 338 | |||||
As triazines a is group of chemical compounds called the basic structure of an aromatic heterocycle , the three nitrogen atoms in the six-membered ring system contains.
The unsubstituted parent compounds are of little importance. Of the substituted derivatives , the symmetrical 1,3,5-triazines, in which the six-membered ring consists of three alternating carbon and nitrogen atoms, are particularly important as versatile building blocks for further syntheses .
history
The first commercially used triazine was the fungicide anilazine (1953). This was followed by the triazine herbicides from the group of chlorodiaminotriazines, for which JR Geigy (today: Novartis ) applied for a patent in 1954 .
The methylmercaptodiaminotriazine (Triatryne) and the methoxydiaminotriazine (Triatone) were added later.
properties
The cyclic trimer of hydrocyanic acid 1,3,5-triazine only decomposes above 600 ° C into hydrocyanic acid, nitrogen and carbon dioxide .
use
Several triazines, such as atrazine and simazine , were widely used as effective herbicides, especially in maize cultivation, but are now banned in the EU due to their persistence , which is hazardous to groundwater . The environmental behavior ( bioaccumulation , adsorption , toxicity ) is very different depending on the chemical structure and side chain.
Numerous other important chemical intermediates also contain the triazine structure. For example, many reactive dyes contain chlorine- or fluorine-substituted triazines as “reactive anchors”: the chromophore is bound to the triazine ring, which then reacts with an OH group of the cellulose (cotton) during the dyeing process under alkaline conditions with HCl cleavage. The dye is bound to the cotton fiber via a covalent chemical bond , which results in a high level of washfastness.
Derivatives (selection)
- Acetoguanamine
- Atrazine
- Cyanazine
- Cyanuric chloride
- Cyanuric fluoride
- Cyanuric acid
- Cyanuric triazide
- Cybutryn
- Desmetryn
- Prometon
- Prometryn
- Propazine
- Simazine
- Terbumetone
- Terbuthylazine
- Terbutryn
- Trisbiphenyltriazines
- melamine
Individual evidence
- ↑ a b c d e f g h Entry on triazines. In: Römpp Online . Georg Thieme Verlag, accessed on March 4, 2014.
- ↑ a b Neunhoeffer, H .; Clausen, M .; Voetter, H.-D .; Ohl, H .; Krueger, C .; Angermund, K .: 1,2,3 ‐ Triazine, III Synthesis of N ‐ aminopyrazoles and their oxidation to 1,2,3 ‐ triazines. Molecular structure of 1,2,3 ‐ triazine in Liebigs Ann. Chem. 1985, 1732-1751, doi : 10.1002 / jlac.198519850903 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of 1,2,3-triazine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on July 28, 2016, is shown, which is derived from a self-classification by the distributor .
- ↑ Data sheet s-Triazine, 97% from Sigma-Aldrich , accessed on November 6, 2013 ( PDF ).
- ↑ Patent US2720480 : Fungicidal compositions and method of using same. Applied July 17, 1953 , published October 11, 1955 , Applicant: Ethyl Corp., Inventor: Calvin N. Wolf.
- ↑ Patent US2891855 : Compositions and methods for influencing the growth of plants. Registered on January 12, 1955 , published on June 23, 1959 , applicant: JR Geigy AG, inventor: Enrico Knusli.
- ^ L. Roth, U. Weller: Hazardous chemical reactions , entry for 1,3,5-triazine, status 72. Supplementary delivery 3/2014, ecomed Verlag Landsberg / Lech, ISBN 978-3609195872 .
- ↑ R. Kornmayer, B. Streit: Adsorption and enrichment of atrazine and its degradation products on river water sediment . In: Arch. Hydrobiol. Suppl. 55, 1978. pp. 186-210.
- ^ B. Streit: Uptake, accumulation and release of organic pesticides by benthic invertebrates. 3. Distribution of 14 C-atrazine and 14 C-lindane in an experimental 3-step food chain microcosm . Arch. Hydrobiol./ Suppl. 55: 374-400 (1979).




