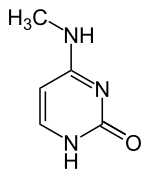
N 4 -methylcytosine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | N 4 -methylcytosine | ||||||||||||
| other names |
4-methylamino-pyrimidin-2-one |
||||||||||||
| Molecular formula | C 5 H 7 N 3 O | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 125.13 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
N 4 -Methylcytosine is a heterocyclic organic compound with a pyrimidine backbone. It is a derivative of the nucleobase cytosine , whichis methylated at the amino group . It occurs as a component of the nucleoside N 4 -methylcytidine (m 4 C) in RNA , and also in bacterial DNA .
The dimethylated variant is N 4 , N 4 -dimethylcytosine .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Melanie Ehrlich, Geoffrey G. Wilson, Kenneth C. Kuo, Charles W. Gehrke: “ N 4 -Methylcytosine as a Minor Base in Bacterial DNA”, J Bacteriol , 1987 , 169 (3), pp. 939-943 ( PMC 211883 (free full text); PMID 3029036 ).
- ↑ Melanie Ehrlich, Miguel A. Gama-Sosa, Laura H. Carreira, Lars G. Ljungdahl, Kenneth C. Kuo, Charles W. Gehrke: "DNA methylation in thermophilic bacteria: N 4 -methylcytosine, 5-methylcytosine, and N 6 -methyladenine ", Nucleic Acids Research , 1985 , 13 (4), pp. 1399-1412 ( doi : 10.1093 / nar / 13.4.1399 ; PMC 341080 (free full text); PMID 4000939 ).