N 6 -Glycinylcarbamoyladenosine
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
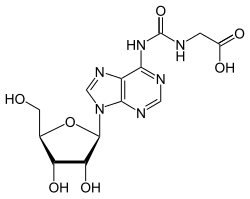
|
|||||||
| General | |||||||
| Surname | N 6 -Glycinylcarbamoyladenosine | ||||||
| other names |
|
||||||
| Molecular formula | C 13 H 16 N 6 O 7 | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 368.31 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
N 6 -Glycinylcarbamoyladenosine (g 6 A) is a rare nucleoside and occurs in the tRNA . It consists of β- D- ribofuranose (sugar) and a substituted adenine . It differs from adenosine by adding a glycine linkedvia a carbamate .
literature
- MP Schweizer, K. McGrath, L. Baczynskyj: "The isolation and characterization of N- [9- (beta-D-ribofuranosyl) -purin-6-ylcarbamoyl] glycine from yeast transfer RNA", Biochem. Biophys. Res. Commun. , 1970 , 40 (5), pp. 1046-1052 ( PMID 5503779 ).
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Patrick A. Limbach, Pamela F. Crain, James A. McCloskey: "Summary: the modified nucleosides of RNA", Nucleic Acids Research , 1994 , 22 (12), pp. 2183-2196 ( doi : 10.1093 / nar / 22.12 .2183 , PMC 523672 (free full text), PMID 7518580 ).
Web links
- Modification Summary of N 6 -Glycinylcarbamoyladenosine in the Modomics database, accessed January 14, 2014.