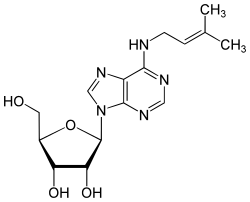
N 6 -isopentenyladenosine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | N 6 -isopentenyladenosine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 15 H 21 N 5 O 4 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 335.36 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
N 6 -isopentenyladenosine (i 6 A, riboprin ) is a rare nucleoside and occurs in the tRNA . It consists of β- D- ribofuranose (sugar) and N 6 -isopentenyladenine . It differs from adenosine by adding an isopentenyl group on the amino group . The adenosine is converted with isopentenyl pyrophosphate by means of the tRNA isopentenyl transferase to form N 6 -isopentenyl adenosine. Like N 6 -threonylcarbamoyladenosine , it is found in addition to the anticodon at position 37 in tRNA in both bacteria and eukaryotes .
literature
- Michel Laloue, Claude Terrine, Jean Guern: "Cytokinins: Metabolism and Biological Activity of N 6 - (Δ 2 -Isopentenyl) adenosine and N 6 - (Δ 2 -Isopentenyl) adenine in Tobacco Cells and Callus", Plant Physiol. , 1977 , 59 , pp. 478-483 ( full text ).
- Maurizio Bifulco, Anna Maria Malfitano, Maria Chiara Proto, Antonietta Santoro, Maria Gabriella Caruso, Chiara Laezza: "Biological and Pharmacological Roles of N6-Isopentenyladenosine: An Emerging Anticancer Drug", Anti-Cancer Agents in Medicinal Chemistry , 2008 , 8 (2 ), Pp. 200-204 ( PMID 18288922 , doi : 10.2174 / 187152008783497028 ).
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Patrick A. Limbach, Pamela F. Crain, James A. McCloskey: "Summary: the modified nucleosides of RNA", Nucleic Acids Research , 1994 , 22 (12), pp. 2183-2196 ( doi : 10.1093 / nar / 22.12 .2183 , PMC 523672 (free full text), PMID 7518580 ).
- ↑ Larry K. Kline, Fritz Fittler, Ross H. Hall: “ N 6 - (Δ 2 -Isopentenyl) adenosine. Biosynthesis in Transfer Ribonucleic Acid in Vitro “, Biochemistry , 1969 , 8 (11), pp. 4361-4371 ( PMID 4311031 ; doi : 10.1021 / bi00839a021 ).
- ↑ Nadja Rosenbaum, Malcolm L. Gefter: “Δ 2 -Isopentenylpyrophosphate: Transfer Ribonucleic Acid Δ 2 -Isopentenyltransferase from Escherichia coli . Purification and Properties of the Enzyme ", J. Biol. Chem. , 1972 , 247 (18), pp. 5675-5680 ( PMID 4341485 ; Abstract ; PDF ).
- ↑ BC Persson, B. Esberg, O. Olafsson, GR Björk: “Synthesis and function of isopentenyl adenosine derivatives in tRNA”, Biochimie , 1994 , 76 (12), pp. 1152-1160 ( PMID 7748950 , doi : 10.1016 / 0300 -9084 (94) 90044-2 ).
Web links
- Modification Summary of N 6 -Isopentenyladenosine in the Modomics database, accessed January 13, 2014.