Cancer immunotherapy
Cancer immunotherapy is the name for different methods of immunotherapy for the treatment of cancers .
The classic treatment methods for cancer are surgical tumor removal ( resection ), chemotherapy and radiation therapy . Often two or even all three forms of therapy are used simultaneously on one patient. The latter two methods have significant cytotoxic side effects . The therapeutic index is very low during chemotherapy, so that a high dosage - which would be conducive to enhancing the effect - is usually excluded. If, however, not all cells of the tumor and its metastases are destroyed ( eradicated ) during therapy , further treatment is made significantly more difficult by the development of resistance . For years, research has therefore been carried out on new therapeutic methods that have the highest possible selective effect against cancer cells. The various approaches of cancer immunotherapy have a very promising potential here, which - for example in antibody therapy - has also found its way into clinical practice.
In oncology, a distinction is made between active and passive vaccination for the different therapeutic approaches . In the active immunization of the patient gets cancer vaccines administered , which in his immune system an immune response should trigger. The immune response should ideally lead to the death of the tumor cells or at least to delayed tumor growth. In contrast, with passive immunization, the patient receives antibodies or antibody fragments . These are supposed to bind selectively to tumor cells and thus lead to their demise. In adoptive immunotherapy, leukocytes are taken from the patient , cultivated ex vivo and then re- injected into the patient .
In the area of passive immunization, several approved antibodies against cancer are already in clinical use. A number of drugs for specific active immunization ( tumor vaccination or cancer vaccination ) in the area of cancer indications are still in clinical development .
The HPV vaccines approved in the European Union since September 2006 are not actually cancer immunotherapy. These vaccines are used preventively to immunize against human papillomavirus (HPV), which can cause certain types of cancer, especially cervical cancer .
| Cancer immunotherapy | ||||
| passive immunization | active immunization | |||
| unspecific | specific | unspecific | specific | |
| Cytokines | Antibodies (with and without conjugate) | Slotted snail hemocyanin | Vaccine from killed tumor cells | |
|
Lymphokine-activated killer cells or cytokine-induced killer cells |
adoptive transfer of T lymphocytes | Freund's adjuvant | Cell extract vaccines | |
| Peginterferon α | Bacillus Calmette-Guérin | Vaccines based on antigens | ||
Classification of cancer immunotherapy with examples.
Cancer and the immune system
Immune surveillance
The theory of immune surveillance ( immunosurveillance ) assumes that the immune system is not only active against foreign pathogens, but also against the body's own degenerate cells. An intact immune system - so the conclusion - is therefore an important element in an organism in order to avoid cancer.
The two examples below make clear what effects a permanently weakened immune system can have in humans.

The human immunodeficiency virus (HIV) destroys CD4 + - T-lymphocytes . These cells , which are part of the white blood cells ( leukocytes ) , play an important role in the immune response . AIDS patients (AIDS = acquired immunodeficiency syndrome = "acquired immunodeficiency syndrome") therefore have a weakened immune system. As a result of this weakened immune system, various cancers, such as lymphoma , Kaposi's sarcoma and cervical carcinoma , are common complications in AIDS patients.
Patients who have received a solid donor organ (such as a kidney or liver ) are given immunosuppressants to prevent the foreign organ from being rejected . These are drugs that reduce the functions of the immune system. Statistically speaking, these patients are three times more likely to develop cancer than comparison groups of the same sex and age group. After ten years of immunosuppression, the chance of developing cancer is 20%. This is especially true for cancers that are of viral origin, such as Kaposi's sarcoma (trigger: human herpes virus 8 ), Hodgkin's disease and non-Hodgkin's lymphoma ( Epstein-Barr virus ), liver cancer ( hepatitis C and hepatitis -B virus ), as well as cervical cancer , vulvar cancer , vaginal cancer , penile cancer and other cancers caused by human papillomavirus . But cancers such as colorectal cancer , kidney cancer , bladder cancer , thyroid cancer , multiple myeloma , leukemia and malignant melanoma - which according to the current state of knowledge are not triggered by viruses - are also increasing significantly. In some cases, regression of the malignancies, for example malignant lymphomas , was observed after the immunosuppression was discontinued .
In both groups - AIDS patients and patients on immunosuppressants after transplants - high similarities were found in terms of cancer risk, with the cause of the increased cancer rate being essentially the weakened immune system.
The exact mechanisms are not yet fully understood, but immune surveillance plays a crucial role in this.
The thesis of immune monitoring, i.e. the direct and constant fight against spontaneously occurring tumors by the immune system and tumor development as a result of weaknesses or disorders of the immune system, is still controversially discussed to this day. On the other hand, it is generally recognized that the immune system is in principle able to recognize degenerate cells and to fight them successfully. As will be shown later, the immune system does not offer complete, but rather inadequate protection against tumor diseases, which is demonstrated by the fact that people with a completely intact immune system can also develop cancer. The use of the existing mechanisms of the immune system are the starting point for a number of strategies for the immunological therapy of cancer diseases.
Immune Escape
It is actually not surprising that the immune system can “fail” in its defense against cancer cells in completely healthy, immune-competent people. After all, tumors form from the body's own cells in a wide variety of tissues that are “out of control”. Accordingly, tumor cells carry the self-antigen on their cell surface , which they identify as “belonging to the body”. This is a completely different situation than with alien "intruders" ( pathogens ), who are relatively easy to recognize by the immune system due to their strangeness. Oncologists therefore do not speak of a “failure of the immune system” when it comes to cancer. On the other hand, an overly sharp immune system that would respond to minimal cellular changes would result in a number of autoimmune diseases .
Many tumor cells do not express mutated peptides ( tumor-specific antigens ) on their surface. This would be an essential prerequisite for the immune system to recognize the degenerated cells as such and destroy them. But even with tumor cells that present antigens on their cell membrane , there is in many cases no adequate immune response. The reason here is that the tumor cells can evade the immune system in various ways. One speaks therefore of the so-called immune escape (" immune escape "). For example, the peptides presented on the MHC-I complex can be changed by mutation. The reduction in the expression of the main histocompatibility complex (MHC) is also, for example, a reaction of the cancer cells. They can also protect themselves by secreting certain immunosuppressive cytokines . The antigen shedding , the dropping of antigens from the outer membrane is to escape, a measure of the cancer cells to the immune system. Some cancer cells are able to express Fas ligands (FasL = Fas ligand; CD95) on their cell surface. If FasL binds to the Fas receptor (FasR), the cancer cells can trigger programmed cell death ( apoptosis ) in the FasR-bearing lymphocytes . This process is called a tumor counterattack (German: "tumor counterattack").
Immunoediting
There is no special strategy or even an independent “intelligence” behind the adaptation of the cancer cells. They are a result of mutation (by the cancer cells themselves) and selection (by the immune system). The best adapted cells survive and continue to multiply ( Survival of the Fittest ). The comparatively high rate of proliferation and mutation of the degenerate cells is advantageous for their evolution. The defense mechanisms used by cancer cells are also present in healthy cells, for example to prevent autoimmunity . The immune system thus does two things in cancer cells: on the one hand, the organism is protected by destroying the degenerate cells and, on the other hand, the tumor is “formed” through the selection process. Since the term immune surveillance, which only looked at the protective function of the immune system, no longer adequately does justice to this expanded thesis, one speaks of immunoediting since the beginning of the 21st century . Tests with transplanted tumors in mice provide evidence for the thesis of immunoediting , i.e. the selection process of non-immunogenic tumor cells . Tumors that were generated in immunocompetent mice with 3-methylcholanthrene (MCA) and that could thus be “shaped” by the mouse's immune system through the selection process, were rejected significantly less often during transplantation into other mice than tumors from immunodeficient mice. Due to the restricted immune system, the tumors from the immunodeficient mice were less influenced by selection processes and thus more immunogenic . The immunoediting is divided into three phases: elimination, balance ( equilibrium ) and escape ( Escape ). The elimination phase corresponds to the original concept of immune surveillance, in which cancer cells are destroyed by the immune system. In the equilibrium phase, after almost complete destruction of the cancer cells, an immune-mediated latency sets in. In the last phase, the escape , the tumor escapes the immune surveillance and only becomes clinically visible in this phase.
The goals of cancer immunotherapy
As was shown at the beginning, the immune system plays an important role in the development or prevention of cancer. But even afterwards, when cancer actually breaks out through an immune escape , the immune system has a major influence on the course of the disease. This is shown, among other things, by the fact that the activity of the immune system against cancer cells is an important factor for the prognosis in cancer patients. For example, it has been shown that cell-mediated immunogenicity to tumor-associated antigens (TAA) is a better indicator of survival than staging , grading and status of the lymph nodes . In patients with breast cancer at an early stage, the survival rate is significantly higher if they show a natural humoral immune response against epithelial mucin-1 of the tumor cells. In 2003 over 100 ovarian carcinomas were examined immunohistologically in a study . A significant correlation between progression-free survival or overall survival and the infiltration of the tumor tissue with T cells (TIL = tumor infiltrating lymphocytes ) could be established. The five-year survival rate for patients with T-cell infiltrated tumors was 38%, while it was only 4.5% for patients without T-cells in the tumor. Similar correlations have been found, for example, for malignant melanoma , bladder cancer , colorectal cancer , prostate , rectal cancer , and neuroblastoma .
The inadequacies and weaknesses of the immune system against cancer cells described in the previous paragraph are the starting point of cancer immunotherapy. Several strategies, some of them very different, were developed to fight cancer cells directly or indirectly via the immune system. The common goal of all these approaches is to use the immune system to destroy cancer cells. This can happen , for example, through unspecific stimulation of the immune system, which results in an increase in the most important cytotoxic cells . The "marking" of tumor cells with monoclonal antibodies to trigger an immune reaction is a cancer immunotherapy procedure, even if the treatment with antibodies is incorrectly referred to as "chemotherapy", and not just colloquially.
Unfortunately, the currently established treatment regimens - with all the indisputable therapeutic successes - have the opposite effect on the immune system: Both chemotherapeutic and radiotherapeutic measures weaken the immune system. The proliferation and the function of lymphocytes is clearly restricted after chemotherapy. For example, there are significantly fewer T cells in the bone marrow of patients after adjuvant (supportive) systemic chemotherapy for breast cancer, and the number of activated NK cells is also reduced over a longer period of time.
Tumor antigens
- → see main article tumor antigen
Tumor antigens are fragments of proteins produced in tumors. These fragments are located on the outer cell membrane of the tumor cells, in the cell plasma and in the cell nucleus. The tumor antigens arise as a result of the genome changed in cancer cells or as a result of a change in gene expression ("switching on and off" genes ). These changes can result in new gene products that are foreign to the body or proteins that are normally only present in the embryonic development phase , for example . Often certain proteins - also present in healthy cells of the body at the time of the illness - are produced ( overexpressed ) in large quantities .
Almost all previously known tumor antigens are presented on the cell membrane via the MHC-I complex ( antigen presentation ). The protein fragments presented there consist of about nine to ten amino acids. The differences in the structure of the antigen or in the frequency of its expression - compared to a normal cell - make them potential target structures ( targets ) for effector cells of the immune system and antibodies. Cytotoxic T cells can recognize the tumor antigens presented via the MHC-I complex via their T cell receptor with the aid of the CD8 receptor and, if necessary, destroy the target cell with the tumor antigen.
As a target structure, the tumor antigens are the basis for most concepts in cancer immunotherapy. Over 2000 tumor antigens are known to date. The ideal tumor antigen - which does not exist in this form - would have the following properties:
- It will only be expressed on the cell membrane by cancer cells. In contrast, it is not present in healthy cells. In such cases one speaks of a tumor-specific antigen (TSA).
- It consists of peptide fragments that are presented on the cell membrane via the MHC-I complex and are recognized by T cells in the MHC-restricted mode.
- Expression on the cell surface should be guaranteed over the entire cell cycle and at the highest possible density and should not be downregulated .
- The tumor antigen is expressed by all cancer cells in all cancer diseases.
In reality it looks different. Most tumor antigens are not tumor-specific (TSA), but tumor-associated (TAA). This means that they are also expressed by healthy cells. However, the tumor antigens are overexpressed in many tumors. Structural changes in the protein sequence can also occur through mutations in the genome. Many tumor antigens only occur in certain types of tumor and there often only in certain cases. For example, HER2 / neu is only overexpressed in 20 to 25% of all breast cancer tumors, which is why therapy with the HER2 / neu-specific antibody trastuzumab only makes sense in these cases . In addition, tumor cells can shut down antigen presentation via the MHC-I complex , for example as a result of the immune escape .
The identification of tumor antigens with high immunogenicity , which come as close as possible to the ideal picture shown above, is one of the greatest challenges for tumor immunology and the key to successful specific immunizations. A rationale in identifying tumor antigens is that their perception by the immune system is an indicator of their relevance in an anti-tumor response. In the past, tumor antigens were essentially identified by analyzing the anti-tumor response in patients. For this purpose, either the peripheral or tumor-infiltrating lymphocytes (TIL) were examined or the humoral immune response was analyzed.Through the knowledge gained in the context of the human genome project and with the help of improved analytical methods, reverse immunology has been established as a powerful high-throughput method for the identification of tumor antigens for several years .
Passive cancer immunotherapy
Monoclonal antibodies
- → see main article Antibodies and Monoclonal Antibodies
Antibodies are protein structures that, with the help of the lock and key principle, are able to recognize antigens and adhere to them. This also applies to tumor antigens as target structures. In the human body, antibodies are produced by differentiated B lymphocytes , i.e. plasmablasts and plasma cells . Each B-lymphocyte produces a different antibody. However, they only differ in the variable part that matches the antigen epitope - the so-called paratope - at the upper end of the Y structure. If antibodies bind to an antigen epitope of a cell via their paratope, macrophages , for example, recognize this immune complex and subsequently try to destroy the cell marked in this way.
The body's own polyclonal antibodies only play a very minor role in the fight against cancer cells. Most cancer cells do not present antigens that are sufficiently modified for the body's own antibodies to bind to them in sufficient numbers. If the polyclonal antibodies were more sensitive, they would also increasingly bind to healthy cells and trigger autoimmune reactions there. Monoclonal antibodies, on the other hand, are completely identical in their structure and only target one epitope of an antigen.
Antibodies play an important role in cancer diagnosis in particular. For example, in immunoscintigraphy, monoclonal antibodies are marked with radionuclides that can be tracked in a patient's body using SPECT ( radiotracer ). If the monoclonal antibodies marked in this way accumulate in certain organ tissues, this can be an indication of metastasis.
After rituximab was approved by the FDA in the United States in 1997, the first therapeutic monoclonal antibody (against B-cell non-Hodgkin lymphoma) was launched. A year later, trastuzumab was approved for use in metastatic breast cancer. Since then, other monoclonal antibodies against other forms of cancer have been developed and approved for their treatment.
The effect of monoclonal antibodies is based on the binding to target structures that are located on the surface of the target cell. You can work either directly or indirectly. With direct action, the antibodies can trigger an intracellular signal cascade in the cancer cell by cross-linking the tumor antigen. This can result in an anti-proliferative effect or an immediate apoptosis of the cell. Rituximab, for example, can trigger apoptosis by cross-linking the tumor antigen CD20 . The cross-linking of tumor antigens such as CD22, CD33 or HLA II with antibodies, however, has an anti-proliferative effect in the target cells. Monoclonal antibodies can also act on cancer cells by blocking certain ligands. Trastuzumab blocks the Her2 / neu receptor, which disrupts the signal chain to the epidermal growth factor and inhibits proliferation. As a result, tumor growth slows down.
The indirect mechanisms of action of the monoclonal antibodies are based on the Fc receptor binding site (Fc = fragment crystallizable ) - the lower part of the Y structure. This activates the effector functions. These include complement-dependent cytolysis ( Complement Dependent Cytolysis , CDC) and antibody-dependent cell-mediated cytotoxicity ( antibody dependent cellular cytotoxicity , ADCC). In CDC, complement fixation (CF) triggers the complement cascade , which leads to lysis of the target cell. However, it is still unclear what significance, or what part, the CDC has for therapeutic success. In ADCC, antibodies direct cytotoxic effector cells, such as NK cells, which themselves do not have any antigen specificity, to the cancer cell via the Fc part. The g chain of the Fc part causes the signal transduction to the cytoplasm of the effector cell. The effector cells release lytic granules , consisting of perforin and granzyme , on the cancer cells , which lead to apoptosis of the cancer cells. For many therapeutic antibodies, such as rituximab, alemtuzumab , trastuzumab or cetuximab , the ADCC is the most important mechanism of action. Phagocytic cells such as macrophages can also be activated via the Fc part of an antibody and phagocytize the cell marked with the antibody. In general, the indirect mechanisms of action are more important in tumor therapy than the direct ones.
The therapeutic limits of monoclonal antibodies
A general problem for the therapeutic effectiveness of the monoclonal antibodies is the ability of the antibodies to bind to the cancer cells. Even with cancer cells that present sufficient tumor antigens, the binding rate is often not sufficiently high. Direct cell damage by associated monoclonal antibodies is the rare exception. The normal case is the triggering of an immune reaction by the resulting immune complex, which - as in the case of the body's own polyclonal antibodies - activates NK cells , macrophages, lymphocytes and granulocytes to destroy the cells marked in this way. For the treatment of larger solid tumors, monoclonal antibodies are largely unsuitable. In these cases there is a problem with distribution and reach. The ratio of tumor cells with corresponding tumor antigens to monoclonal antibodies is too unfavorable here to mark sufficient tumor cells and to kill them by immune defense cells, which are also insufficient in number. With a molecular mass of around 150 kDa , antibodies are generally restricted in their tissue penetration and can only inadequately penetrate deeper layers in solid tumors. Antibody fragments such as Fab , F (ab) 2 or scFv have considerable advantages here because of their significantly smaller size. However, they are also eliminated from the body much faster. The patient's immune system can produce anti-antibodies against murine and chimeric antibodies . If a therapy cycle is repeated, the effectiveness may therefore be reduced. This is true even for humanized and human antibodies.
The immune escape described above also means that not enough antibodies reach the tumor. For example, as a result of the antigen shedding , the tumor cells release tumor antigens and release them into the bloodstream. There they bind to the antibodies and make them ineffective.
For some forms of cancer, such as leukemia or non-Hodgkin lymphoma , which do not form tumors, so that the concentration and distribution problem for the monoclonal antibodies does not exist, the best therapeutic results are achieved with monoclonal antibodies. In the case of solid tumors, therapy with the corresponding monoclonal antibodies is usually carried out after surgical removal of the tumor, chemotherapy or radiation in order to destroy individual free tumor cells in the body and thereby prevent the formation of metastases.
Bispecific antibodies
- → see main article on bispecific antibodies
Antibody conjugates
- → see main article immunoconjugate
In order to increase the relatively weak effect of the monoclonal antibodies in cancer diseases, various drug targeting strategies have been developed in order to use the antibodies as carriers for more potent drugs. In this case, the antibody serves as a “transport vehicle” in order to deliver the active substance as specifically as possible only to tumor cells and to kill them in a targeted manner. The healthy tissue should be spared as much as possible, whereby the side effects can be reduced. Radionuclides , toxins (for example diphtheria toxin ), cytostatics or even cytokines (such as interleukin-2 ) are bound (conjugated) to the corresponding antibody as active ingredients . One speaks in these cases of "armed antibodies" (English. Armed antibodies ). In the case of cytokines, one speaks of immune cytokines .
In principle, it is possible to bind highly potent active ingredients to antibodies which, if freely administered systemically, would lead to unacceptable side effects due to their high toxicity. The conjugate of antibodies or antibody fragments with the active ingredient can be viewed as a prodrug which, when administered systemically, has the lowest possible toxicity and only unfolds its full effect at the site of action, the tumor or an individual cancer cell.
The antibody conjugates consist of three components:
- the antibody or antibody fragment,
- the active ingredient and
- the connecting piece ( linker ) between antibody and active ingredient.
- → For detailed information reference is made to the respective main article Chemoimmunkonjugat , immunotoxin and radioimmunotherapy directed
Active cancer immunotherapy
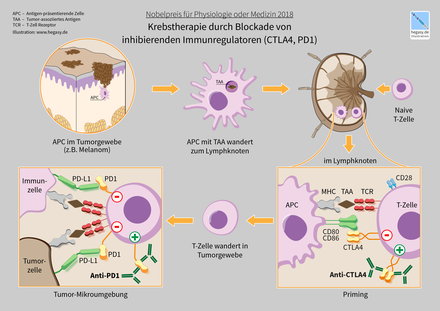
Active immunotherapy is usually divided into specific and non-specific cancer immunotherapy. In unspecific immunotherapy, the immune system is stimulated in its entirety by the administration of a drug. With specific immunotherapy, only certain target cells of the immune system are stimulated. An example of a promising active immunotherapy is the administration of so-called immune checkpoint inhibitors , such as CTLA-4 blocking antibodies or PD-1 / PD-L1 specific antibodies in melanoma. Both checkpoint inhibitors lead to a targeted activation of the immune system and have achieved promising results in recently published clinical studies.
Slotted snail hemocyanin
- → see main article slotted snail hemocyanin
Keyhole limpet hemocyanin (KLH) is the oxygen transport protein of the large California keyhole limpet ( Megathura crenulata ). The protein of this water snail from the family of keyhole limbs (Fissurellidae), which lives at adepth of 40 m,is based on the copper complex hemocyanin , whichtriggers a strong immune responsein mammals - whose oxygen transport protein hemoglobin is based on an iron complex . It is approved under the international non-proprietary name Immunocyanin (brand name Immucothel ) in the Netherlands , Austria and South Korea , but not in Germany, for redicative prophylaxis in bladder cancer after transurethral resection .
The immune-stimulating effect of hemocyanin in mammals was first established in the mid-1960s. From 1967 onwards, KLH was used in humans for diagnostic purposes in immunocompetence tests. The US urologist Carl A. Olsson also used KLH for immunodiagnostic purposes in patients with superficial bladder cancer. As a result, he noted that the patients who were tested developed significantly fewer relapses than the untested patients.
The immune-stimulating effect of KLH can be explained by the secondary structure of hemocyanin, which is partly similar to that of antibodies, histocompatibility molecules or Bence Jones proteins . There are also epitope-like sequences on the surface of the protein.
KLH is also used as a carrier for haptens . In this case, non-immunogenic epitopes are linked with the immunogenic KLH to form an immunoconjugate in order to achieve an immunogenic effect on the epitope. The molecules of many antigen epitopes are too small to be recognized by the immune system. This method can also be used to present antigens to the immune system as "hostile" that the body would otherwise tolerate. In the case of large carrier molecules such as KLH, several different antigens can also be bound to the surface of the carrier.
Bacillus Calmette-Guérin
- → see main article Bacillus Calmette-Guérin
Bacillus Calmette-Guérin (BCG) was obtained from bovine tubercle bacilli at the beginning of the 20th century and used as a live vaccine against tuberculosis . In 1959, mice with transplanted tumors were found for the first time to have a positive effect on tumor regression when infected with BCG. The infection increased the activity of the reticulohistiocytic system and the number of antibodies in the test animals.
With direct injection into tumors, regression of the tumor could be observed in many cases. The treatment attempts for metastatic melanoma were promising , where the systemic administration of BCG also led to a regression of the metastases and the survival rate of the patients treated in this way was significantly increased. However , the positive results could not be confirmed in randomized, controlled studies .
In 1976, positive results in the treatment of superficial bladder cancer with BCG were published for the first time . BCG was injected directly into the urinary bladder (intravesically). The therapeutic efficacy in the treatment of superficial bladder cancer - the tumor is limited to the inner lining of the urinary bladder - has been proven in a large number of clinical studies . BCG therapy is the gold standard for this disease . According to several authors, this is the most successful cancer immunotherapy to date. BCG is clearly superior to any chemotherapeutic agent. The likelihood of tumor recurrence is only half as high compared to intravesical chemotherapy. In over 80% of cases, eradication , i.e. complete elimination of the tumor, is achieved.
The exact mechanism of action of BCG is not yet clear. The bladder is a largely isolated and closed organ in which BCG can reach a very high local concentration and cause a complex, long-lasting local activation of the immune system. As a result of the local activation, various cytokines are released into the urine or to the bladder tissue. At the same time , granulocytes and monocytes infiltrate the bladder wall. Dendritic cells release TNF-α and interleukin-12 (IL-12). The release of these cytokines in turn activates cells of the innate immune system (STIL), which means that IL-12 receptor-expressing NK cells multiply (proliferate).
The most important side effects of therapy with BCG are: cystitis (inflammation of the urinary bladder), pollakiuria (frequent urination), hematuria (blood in the urine) and fever . In studies involving more than 500 patients, no BCG-related death is known.
Adoptive cell transfer
Tumors can also be treated with adoptive cell transfer . For this therapy, for example, autologous T cells (i.e. those derived from the patient himself) are used which have been equipped with a tumor antigen- specific T cell receptor or a chimeric antigen receptor (CAR). This gives the T cells a new specificity directed against the tumor. If the tumor cells present the antigens corresponding to the receptor on their surface, they can be eliminated by the T cells. Therapy with tumor-infiltrating lymphocytes (TILs) or with antigen-laden dendritic cells is also possible. The latter serves to induce an immune response in vivo and activate tumor antigen-specific T cells in the patient's body.
history
The idea of influencing the immune system so that it is able to recognize and destroy neoplasms is very old. The first reports of tumor regressions after infectious diseases date back to 1550 BC. In the Ebers papyrus , the recommended treatment for swellings (tumors) was a cataplasm (a kind of pulp containing a mixture of plant powders, seeds and other medicinal substances), followed by an incision in the tumor. Such treatment leads to infection of the tumor.
The first immunological experiments of the modern age were carried out in 1777 by James Nooth , physician to Edward Augustus, Duke of Kent and Strathearn , and member of the Royal Collage of Surgeons . Nooth implanted foreign tumor tissue several times in small incisions in his arm in order to achieve cancer prophylaxis . The implantation only resulted in inflammatory reactions and less pain. A similar result was obtained in 1808 by Jean-Louis Alibert , the personal physician of King Louis XVIII. who had a colleague inject liquid into a breast cancer patient. There was only an inflammatory reaction.
In contrast, the experiments of the American William Coley (1862–1936), who is considered a pioneer of cancer immunotherapy, were much more successful . Coley was a doctor at Memorial Sloan-Kettering Cancer Center in New York City . He had heard of a cancer patient who went into complete remission after a high fever caused by erysipelas ( sore throat , a bacterial infection of the upper layers of the skin and lymphatic system). Coley noted that Robert Koch , Louis Pasteur , Paul von Bruns, and Emil von Behring had also described similar cases after erysipelas. In 1888, for example, Bruns published a summary of his clinical observations on complete sarcoma regressions in spontaneous or artificially induced erysipelas. In 1891 Coley injected a cancer patient - a 40-year-old Italian immigrant who had already had two operations after relapses and only a few weeks to go according to the prognosis - the erysipelas of the species Streptococcus pyogenes directly into the tumor. Coley repeated the injections over several months and the patient's tumor regressed. The patient survived eight years. Coley later used a mixture ( Coley's Toxin ) of killed bacteria of the species Streptococcus pyogenes and Serratia marcescens , together with the still active endotoxins , directly in tumors. With his method, Coley achieved the remarkable cure rate of 10% for soft tissue sarcomas. The response rates varied widely and the side effects were considerable. From 1899 the Parke-Davis Corporation produced Coley’s toxin , which was widely used for the next 30 years. A major reason for its spread was that it was the only systemic cancer therapy until 1934. With the development of radiation therapy and advances in chemotherapy , Coley's toxin was largely forgotten. Parke-Davis ceased production of Coley's toxin in 1952 , and the FDA denied approval as a tested drug in 1962. The exact mechanism of action is still unclear to this day, but is very likely based on the triggering of a cytokine cascade, which results in a specific and unspecific immune response.
In 1909 Paul Ehrlich was the first to formulate the thesis that the immune system can recognize and eliminate tumor cells. In this way, many tumors would be eliminated at a very early stage and the body would be protected from a significantly higher incidence of malignant tumors.
“I am convinced that aberrant germs occur extremely frequently in the colossally complicated course of fetal and post-fetal development, but that fortunately they remain completely latent in the vast majority of people, thanks to the protective devices of the organism. If this did not exist, one could assume that the carcinoma would occur with an almost monstrous frequency "
The American biostatistician Raymond Pearl published an autopsy study in 1929 in which he found a significantly lower cancer rate in patients with tuberculosis. When the Lübeck vaccination accident with the Bacillus Calmette-Guérin (BCG) - a vaccine against tuberculosis - occurred the following year, this meant the temporary end of appropriate therapeutic approaches in oncology. It was not until the late 1950s that Lloyd J. Old took up the idea again and successfully introduced BCG into cancer immunotherapy. From 1969 BCG was then used in clinical practice.
Lewis Thomas (1913–1993) and the later Nobel Prize winner Frank Macfarlane Burnet took up Ehrlich's thesis independently of one another in the 1950s and 1970s and formulated the hypothesis of “immune surveillance” ( immunosurveillance ) in tumor development. Both Thomas and Burnet hypothesized that T cells play an essential role in immunological surveillance.
The immunological surveillance hypothesis was very controversial for a long time and even seemed to have been refuted by experiments with nude mice in the 1970s. Naked mice, also called athymic mice, have a severely restricted immune system due to the lack of a thymus . According to the thesis of immune surveillance, these mice should develop tumors significantly more frequently than mice of the wild type . In comparative experiments with 3-methylcholanthrene - a strong carcinogen that induces sarcomas in mice - no significant difference in the tumor rate between athymic and normal mice was found. A few years later, however, it was found that, contrary to initial assumptions, nude mice are not completely free of T cells and also have a normal number of NK cells. The NK cells in particular can become active against tumor cells on their own.
The definitive evidence of immune surveillance was provided in 2001 by Vijay Shankaran and colleagues. Knockout mice in which the RAG2 and STAT1 genes were switched off had a significantly increased incidence of spontaneous or chemically induced tumor formation. The gene products of RAG2 and STAT1 play an important role in the maturation of T and B cells and in signal transmission after binding to the γ-interferon receptor.
Pierre van der Bruggen and colleagues identified in 1991 at the Ludwig Institute for Cancer Research in Brussels with MAGEA1 ( melanoma antigen family A, 1 ) the first time an epitope malignant origin, which is recognized by T cells. As a result, more than 70 other tumor-associated antigens with immunogenic properties were found , including with cDNA expression cloning .
In March 2015, the two American immunotherapists James P. Allison and Carl H. June were awarded the € 100,000 Paul Ehrlich and Ludwig Darmstaedter Prize for their groundbreaking work on cancer immunotherapy . James Allison pioneered checkpoint inhibition for the treatment of advanced melanoma , while Carl June developed the CART-19 therapy for leukemia .
literature
- German Cancer Research Center (Hrsg.): Insight: weapons of the immune system. (PDF; 3.9 MB) Issue 1/2010
- C. Huber (ed.) U. a .: Cancer immunotherapies. Deutscher Ärzteverlag, 2007, ISBN 3-7691-1212-1
- GC Prendergast and EM Jaffee: Cancer Immunotherapy. Academic Press, 2007, ISBN 0-12-372551-8 (English)
- JP Allison et al. a .: Cancer Immunotherapy. Academic Press, 2006, ISBN 0-12-022489-5 (English)
- ML Disis: Immunotherapy of Cancer. Humana Press, 2006, ISBN 1-58829-564-8 (English)
- JH Finke, RM Bukowski: Cancer immunotherapy at the crossroads. Humana Press, 2004, ISBN 1-58829-183-9 (English)
- PL star u. a .: Cancer vaccines and immunotherapy. Cambridge University Press, 2000, ISBN 0-521-62263-8 (English)
Individual evidence
- ↑ a b S. Lang: Recombinant Parvoviruses in the gene therapy of cancer: vector characterization and analysis of the effectiveness. Dissertation, Ruprecht-Karls-Universität Heidelberg, 2003.
- ↑ a b F. O. Losch: Activation of T lymphocytes by melanoma-specific variant antigen receptors. Dissertation, Albert-Ludwigs-Universität Freiburg, 2001
- ↑ Tumor Immunology. ( Memento of the original from March 22, 2005 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 1.9 MB) Institute for Immunology at the University of Kiel
- ^ WG Land: Immunosuppressive Therapy. Georg Thieme Verlag, 2006, ISBN 3-13-133621-8 , p. 134.
- ↑ a b c C. M. Vajdic and MT van Leeuwen: Cancer incidence and risk factors after solid organ transplantation. In: Int J Cancer 2009, [Epub ahead of print]. PMID 19444916
- ^ A. Kapoor: Malignancy in kidney transplant recipients. In: Drugs 68, 2008, pp. 11-19. PMID 18442297 (Review)
- ^ A. Gutierrez-Dalmau and JM Campistol: Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. In: Drugs 67, 2007, pp. 1167-1198. PMID 17521218 (Review)
- ↑ TE Starzl u. a .: Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. In: The Lancet 8377, 1984, pp. 583-587. PMID 6142304
- ↑ AM Asemissen: Phenotype characterization of reactive T cells from melanoma patients against autologous tumor cells and a new tyrosinase epitope. Dissertation, Humboldt University Berlin, 2004
- ^ AE Grulich: Incidence of cancers in people with HIV / AIDS compared with immunosuppressed transplant recipients: a meta-analysis. In: The Lancet 370, 2007, pp. 59-67. PMID 17617273
- ↑ CM Vajdic and MT van Leeuwen: What types of cancers are associated with immune suppression in HIV? Lessons from solid organ transplant recipients. In: Curr Opin HIV AIDS 4, 2009, pp. 35-41. PMID 19343829 (Review)
- ↑ a b c d G. P. Dunn u. a .: Cancer immunoediting: from immunosurveillance to tumor escape. In: Nat Immunol 3, 2002, pp. 991-998. PMID 12407406 (Review)
- ↑ Tumor-specific targeting of the human natural killer cell line YT by gene transfer of chimeric immunoglobulin T-cell receptors. Dissertation, Humboldt University Berlin, 2005.
- ↑ The Immune System: Function and Significance in Cancer , Cancer Information Service of the German Cancer Research Center (DKFZ), Heidelberg. September 28, 2010. Last accessed September 4, 2014.
- ↑ PG Coulie et al. a .: A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. In: PNAS 92, 1995, pp. 7976-7980. PMID 7644523
- ^ PF Robbins et al. a .: A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. In: J Exp Med 183, 1996, pp. 1185-1192. PMID 8642260
- ↑ T. Wolfel et al. a .: A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. In: Science 269, 1995, pp. 1281-1284. PMID 7652577
- ^ EP Cohen and TS Kim: Neoplastic cells that express low levels of MHC class I determinants escape host immunity. In: Semin Cancer Biol 5, 1994, pp. 419-428. PMID 7703441 (Review)
- ↑ a b I. Algarra u. a .: The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. In: Cancer Immunol Immunother 53, 2004, pp. 904-910. PMID 15069585 (Review)
- ^ PH Black: Shedding from the cell surface of normal and cancer cells. In: Adv Cancer Res 32, 1980, pp. 75-199. PMID 7008543 (Review)
- ↑ HT Khong and NP Restifo: Natural selection of tumor variants in the generation of "tumor escape" phenotypes. In: Nat Immunol 3, 2002, pp. 999-1005. PMID 12407407 (Review)
- ↑ U. Gnad-Vogt u. a .: Pancreatic cancer: EGFR and immunotherapy. In: J Oncology 4, 2006
- ↑ a b V. Shankaran et al. a .: IFNγ prevent prevent primary tumor development and shape tumor immunogenicity. In: Nature 410, 2001, pp. 1107-1111. PMID 11323675
- ↑ D. Nargosen: Antigen-specific T-cell immunity and antigen-presenting cells in malignant diseases. Habilitation thesis, Charité Berlin, 2006
- ↑ a b c L. Zhang u. a .: Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. In: NEJM 348, 2003, pp. 203-213. PMID 12529460
- ↑ a b E. F. Solomayer and a .: Influence of adjuvant hormone therapy and chemotherapy on the immune system analyzed in the bone marrow of patients with breast cancer. In: Clin Cancer Res 9, 2003, pp. 174-180. PMID 12538466
- ↑ JL Mccoy et al. a .: Cell-mediated immunity to tumor-associated antigens is a better predictor of survival in early stage breast cancer than stage, grade, or lymph node status. In: Breast Cancer Res 60, 2000, pp. 227-234. PMID 10930110
- ↑ S. Von Mensdorrf-Pouilly u. a .: Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. In: J Clin Oncol 18, 2000, pp. 547-582. PMID 10653872
- ↑ F. Rilke et al. a .: Prognostic significance of HER-2 / neu expression in breast cancer and its relationship to other prognostic factors. In: J Cancer 49, 1991, pp. 44-49. PMID 1678734
- ↑ WH Clark et al. a .: Model predicting survival in stage I melanoma based on tumor progression. In: J Natl Cancer Inst 81, 1989, pp. 1893-1904. PMID 2593166
- ↑ CG Clemente et al. a .: Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. In: Cancer 77, 1996, pp. 1303-1310. PMID 8608507
- ^ PK Lipponen u. a .: Tumor infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancer. In: Eur J Cancer 29A, 1992, pp. 69-75. PMID 1445749
- ↑ L. Nacopoulou et al. a .: Prognostic significance of histologic host response in cancer of the large bowel. In: Cancer 47, 1981, pp. 930-936. PMID 7226044
- ↑ Y. Naito et al. a .: CD8 + T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. In: Cancer Res 58, 1998, pp. 3491-3494. PMID 9721846
- ^ NA Epstein and LP Fatti: Prostatic carcinoma: some morphological features affecting prognosis. In: Cancer 37, 1976, pp. 2455-2465. PMID 1260728
- ↑ JR Jass: Lymphocytic infiltration and survival in rectal cancer. In: J Clin Pathol 39, 1986, pp. 585-589. PMID 3722412
- ↑ L. Palma et al. a .: Lymphocytic infiltrates in primary glioblastomas and recidivous gliomas. Incidence, fate, and relevance to prognosis in 228 operated cases. In: J Neurosurg 49, 1978, pp. 854-861. PMID 731302
- ↑ a b c Monoclonal Antibodies: Important in Diagnostics and Therapy , Cancer Information Service of the German Cancer Research Center (DKFZ), Heidelberg. June 27, 2014. Last accessed September 4, 2014.
- ↑ DH Kang et al. a .: Significant impairment in immune recovery after cancer treatment. In: Nurs Res 58, 2009, pp. 105-114. PMID 19289931
- ↑ S. Uh u. a .: The effect of radiation therapy on immune function in patients with squamous cell lung carcinoma. In: Chest 105, 1994, pp. 132-137. PMID 7903922
- ↑ M. Tagawa et al. a .: Virology- and immunology-based gene therapy for cancer. In: Cancer Immunol Immunother 55, 2006, pp. 1420-1425. PMID 16691360 (Review)
- ↑ a b c d K. Kokowski: Cellular immune response against the tumor antigens mucin and telomerase in patients with breast cancer. Dissertation, FU Berlin, 2008.
- ↑ a b c R. H. Vonderheide: Telomerase as a universal tumor-associated antigen for cancer immunotherapy. In: Oncogene 21, 2002, pp. 674-679. PMID 11850795 (Review)
- ^ BJ van den Eynde and P. van der Bruggen: T cell defined tumor antigens. In: Curr Opin Immunol 9, 1997, pp. 684-693. PMID 9368778 (Review)
- ↑ LM Weiner u. a .: Monoclonal antibodies for cancer immunotherapy. In: Lancet 373, 2009, pp. 1033-1040. PMID 19304016 (Review)
- ↑ F. Yang and XF Yang: New concepts in tumor antigens: their significance in future immunotherapies for tumors. (PDF; 251 kB) In: Cell Mol Immunol 2, 2005, pp. 331-341. PMID 16368059 (Review)
- ↑ M. Untch u. a .: Adjuvant therapy with trastuzumab in breast cancer patients. In: Dtsch Arztebl 103, 2006, pp. A-3406 / B-2961 / C-2841
- ↑ S. Viatte et al. a .: Reverse immunology approach for the identification of CD8 T-cell-defined antigens: Advantages and hurdles. In: Immunology and Cell Biology 84, 2006, pp. 318-330. PMID 16681829 doi: 10.1111 / j.1440-1711.2006.01447.x (Review)
- ↑ a b c J. D. Gordan and RH Vonderheide: Universal tumor antigens as targets for immunotherapy. In: Cytotherapy 4, 2002, pp. 317-327. PMID 12396831 (Review)
- ↑ a b P. van der Bruggen u. a .: A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. In: Science 254, 1991, pp. 1643-1647. PMID 1840703
- ↑ Y. Kawakami et al. a .: Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. In: J Exp Med 180, 1994, pp. 347-352. PMID 7516411
- ↑ U. Sahin and a .: Serological identification of human tumor antigens. In: Curr Opin Immunol 9, 1997, pp. 709-716. PMID 9368781 (Review)
- ↑ M. Schirle u. a .: Identification of tumor-associated MHC class I ligands by a novel T cell-independent approach. In: Eur J Immunol 30, 2000, pp. 2216-2225. PMID 10940913
- ↑ M. Schwarz: Identification of potentially immunogenic peptides on cells of chronic myeloid leukemia. Dissertation, FU Berlin, 2004
- ↑ JC Castle et al .: Exploiting the mutanome for tumor vaccination. In: Cancer Research 2 (5): 1081-91. PMID 22237626
- ↑ a b D. Schrama u. a .: Antibody targeted drugs as cancer therapeutics. In: Nat Rev Drug Discov 5, 2006, pp. 147-159. PMID 16424916
- ↑ S. Rueckert u. a .: A monoclonal antibody as an effective therapeutic agent in breast cancer: trastuzumab. In: Expert Opinion on Biological Therapy 5, 2005, pp. 853-866. PMID 15952915
- ↑ MJ Glennie and PW Johnson: Clinical trials of antibody therapy. In: Immunol Today 21, 2000, pp. 403-410. PMID 10916144 (Review)
- ↑ D. Shan et al. a .: Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. In: Blood 91, 1998, pp. 1644-1652. PMID 9473230
- ↑ M. Dechant et al. a .: HLA class II antibodies in the treatment of hematologic malignancies. In: Semin Oncol 30, 2003, pp. 465-475. PMID 12939715 (Review)
- ↑ R. Meng u. a .: The evaluation of recombinant, chimeric, tetravalent antihuman CD22 antibodies. In: Clin Cancer Res 10, 2004, pp. 1274-1281. PMID 14977825
- ↑ C. Vitale et al. a .: Engagement of p75 / AIRM1 or CD33 inhibits the proliferation of normal or leukemic myeloid cells. In: PNAS 96, 1999, pp. 15091-15096. PMID 10611343
- ↑ a b c d e C. Kellner: Development and characterization of bispecific antibody derivatives for the immunotherapy of CD19-positive leukemias and lymphomas. Dissertation, Friedrich-Alexander University Erlangen-Nuremberg, 2008.
- ↑ M. Harries and I. Smith: The development and clinical use of trastuzumab (Herceptin). ( Memento of the original from December 2, 2009 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. In: Endocr-Relat Cancer 9, 2002, pp. 75-85. PMID 12121832 (Review)
- ↑ O. Some things u. a .: In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. In: Blood 101, 2003, pp. 949-954. PMID 12393572
- ^ WK Weng and R. Levy: Expression of complement inhibitors CD46, CD55, and CD59 on tumor cells does not predict clinical outcome after rituximab treatment in follicular non-Hodgkin lymphoma. In: Blood 98, 2001, pp. 1352-1357. PMID 11520782
- ↑ OH Brekke and I. Sandlie: Therapeutic antibodies for human diseases at the dawn of the twenty-first century. In: Nat Rev Drug Discov 2, 2003, pp. 52-62. PMID 12509759 (Review)
- ^ A b P. Carter: Improving the efficacy of antibody-based cancer therapies. In: Nat Rev Cancer 1, 2001, pp. 118-129. PMID 11905803 (Review)
- ^ I. Heisler: Importance of cleavable peptide linkers for the function of recombinant saporin-EGF immunotoxins. Dissertation, FU Berlin, 2008
- ^ RK Jain and LT Baxter: Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. In: Cancer Res 48, 1988, pp. 7022-7032. PMID 3191477
- ↑ T. Yokota et al. a .: Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. In: Cancer Res 52, 1992, pp. 3402-3408. PMID 1596900
- ↑ J. Chen et al. a .: Antibody-cytotoxic agent conjugates for cancer therapy. In: Expert Opin Drug Deliv 2, 2005, pp. 873-890. PMID 16296784 (Review)
- ↑ DE Milenic u. a .: Antibody-targeted radiation cancer therapy. In: Nat Rev Drug Discov 3, 2004, pp. 488-499. PMID 15173838
- ↑ D. Baron: Therapeutic use of monoclonal antibodies. In: Zeitschrift Naturwissenschaften 84, 1997, pp. 189–198. doi: 10.1007 / s001140050376
- ^ GC MacDonald and N. Glover: Effective tumor targeting: strategies for the delivery of Armed Antibodies. In: Curr Opin Drug Discov Devel 8, 2005, pp. 177-183. PMID 15782542 (Review)
- ^ RA Reisfeld u. a .: Immunocytokines: a new approach to immunotherapy of melanoma. In: Melanoma Res 7, 1997, pp. 99-106. PMID 9578424 (Review)
- ↑ S. Jaracz et al. a .: Recent advances in tumor-targeting anticancer drug conjugates. In: Bioorg. Med. Chem. 13, 2005, pp. 5043-5054. PMID 15955702 (Review)
- ^ RV Chari: Targeted cancer therapy: conferring specificity to cytotoxic drugs. In: Acc Chem Res 41, 2008, pp. 98-107. PMID 17705444 (Review)
- ^ Semin Oncol. 2015 Jun; 42 (3): 363-377. doi: 10.1053 / j.seminoncol . 2015.02.015 . Epub 2015 Feb 16. Immune Checkpoint Protein Inhibition for Cancer: Preclinical Justification for CTLA-4 and PD-1 Blockade and New Combinations. Baksh K1, Weber J2.
- ↑ a b B. Pfeifer u. a .: Integrative oncology. Verlag Elsevier, Urban & Fischer, 2006, ISBN 3-437-56420-X , p. 239.
- ^ WO Weigle: Immunochemical properties of hemocyanin. In: Immunochem 1, 1964, pp. 295-302. PMID 14250783
- ↑ JE Curtis et al. a .: The human primary immune response to keyhole limpet hemocyanin: interrelationships of delayed hypersensitivity, antibody response and in vitro blast transformation. In: Clin Exp Immunol 6, 1970, pp. 473-491. PMID 4320164
- ↑ FJ Dixon et al. a .: The antibody responses of rabbits and rats to hemocyanin. In: J Immunol 97, 1966, pp. 350-355. PMID 5925706
- ^ MA Swanson and RS Schwartz: Immunosuppressive therapy. The relation between clinical response and immunologic competence. In: NEJM 277, 1967, pp 163-170. PMID 4166031
- ↑ CA Olsson, R. Chute, CN Rao: Immunologic reduction of bladder cancer recurrence rate. In: The Journal of urology. Volume 111, Number 2, February 1974, pp. 173-176, PMID 4810758 .
- ↑ a b J. Schütz: Isolation, sequencing and investigation of physico-chemical properties of structural subunits and functional units of various arthropod and mollusc hemocyanins. Dissertation, Eberhard-Karls-Universität Tübingen, 2000
- ^ R. Roth: Haemocyanin - a strong antigen In: Immunology Spectrum 3, Boehringer GmbH, Mannheim, 1990.
- ↑ J. Stoschek: biosyn drugs: integrative concept in oncology as a goal. In: Dtsch Arztebl 98, 2001, pp. A-1983 / B-1678 / C-1491.
- ↑ C. Huber et al. a. (Editor) Cancer Immunotherapies. Deutscher Ärzteverlag, 2007, ISBN 3-7691-1212-1 , p. 160.
- ↑ LJ Old u. a .: Effect of Bacillus Calmette-Guerin infection on transplanted tumors in the mouse. In: Nature 184, 1959, pp. 291-292. PMID 14428599
- ↑ D. Morton et al. a .: Immunological factors which influence response to immunotherapy in malignant melanoma. In: Surgery 68, 1970, pp. 158-163. PMID 10483463
- ↑ FR Eilber u. a .: Results of BCG adjuvant immunotherapy for melanoma of the head and neck. In: Am J Surg 132, 1976, pp. 476-479. PMID 1015538
- ↑ R. Molife and BW Hancock: Adjuvant therapy of malignant melanoma. In: Crit Rev Oncol Hematol 44, 2002, pp. 81-102. PMID 12399001 (Review)
- ↑ a b I. D. Davis et al. a .: Rational approaches to human cancer immunotherapy. ( Memento of the original from April 11, 2009 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. In: J Leukoc Biol 73, 2003, pp. 3-29. PMID 12525559 (Review)
- ↑ A. Morales et al. a .: Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. In: The Journal of Urology 116, 1976, pp. 180-183. PMID 820877
- ↑ DC Chade et al. a .: Immunomodulatory effects of recombinant BCG expressing pertussis toxin on TNF-alpha and IL-10 in a bladder cancer model. In: J Exp Clin Cancer Res 27, 2008, 78. PMID 19040745 (Open Access)
- ↑ MD Shelly et al. a .: Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumor recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. In: BJU Int 93, 2004, pp. 485-490. PMID 15008714 (Review)
- ↑ MD Shelly et al. a .: Intravesical bacillus Calmette-Guerin versus mitomycin C for Ta and T1 bladder cancer. In: Cochrane Database Syst Rev 3, 2003, CD003231. PMID 12917955 (Review)
- ^ R. De Jager et al. a .: Long-term complete remission in bladder carcinoma in situ with intravesical TICE bacillus Calmette Guerin. Overview analysis of six phase II clinical trials. In: Urology 38, 1991, pp. 507-513. PMID 1836081
- ↑ FM Martin and AM Kamat: Definition and management of patients with bladder cancer who fail BCG therapy. In: Expert Rev Anticancer Ther 9, 2009, pp. 815-820. PMID 19496718
- ↑ a b c H. W. Herr and A. Morales: History of bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. In: The Journal of urology 179, 2008, pp. 53-56. PMID 17997439 (Review)
- ↑ JC Kim and GD Steinberg: The limits of bacillus Calmette-Guerin for carcinoma in situ of the bladder. In: J Urol 165, 2001, pp. 745-756. PMID 11176460 (Review)
- ↑ a b A. Böhle and S. Brandau: Immune mechanisms in bacillus Calmette-Guerin immunotherapy for superficial bladder cancer. In: J Urol 170, 2003, pp. 964-969. PMID 12913751 (Review)
- ↑ CL Amling: Diagnosis and management of superficial bladder cancer. In: Curr Probl Cancer 25, 2001, pp. 219-278. PMID 11514784 (Review)
- ↑ AM Jackson et al. a .: Changes in urinary cytokines and soluble intercellular adhesion molecule – 1 (ICAM-1) in bladder cancer patients after bacillus Calmette-Guerin (BCG) immunotherapy. In: Clin Exp Immunol 99, 1995, pp. 369-375. PMID 7882559
- ↑ GN Thalmann u. a .: Urinary interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guerin. In: J Urol 164, 2000, pp. 2129-2133. PMID 1106194
- ^ S. Prescott et al. a .: Intravesical Evans strain BCG therapy: quantitative immunohistochemical analysis of the immune response within the bladder wall. In: J Urol 147, 1991, pp. 1636-1642. PMID 1593713
- ↑ A. Böhle et al. a .: Effects of local bacillus Calmette-Guerin therapy in patients with bladder carcinoma on immunocompetent cells of the bladder wall. In: J Urol 144, 1990, pp. 53-58. PMID 235918
- ↑ T. Higuchi et al. a .: A possible mechanism of intravesical BCG therapy for human bladder carcinoma: involvement of innate effector cells for the inhibition of tumor growth. In: Cancer Immunol Immunother 58, 2009, p. 1245-1255. PMID 19139883 (Open Access)
- ↑ MD Shelly et al. a .: Intravesical Bacillus Calmette-Guerin in Ta and T1 Bladder Cancer. In: Cochrane Database Syst Rev 4, 2000, CD001986. PMID 11034738 (Review)
- ^ Science. 2006 Oct 6; 314 (5796): 126-9. Epub 2006 Aug 31. Cancer regression in patients after transfer of genetically engineered lymphocytes. Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA .
- ↑ N Engl J Med. 2014 Oct 16; 371 (16): 1507-17. doi: 10.1056 / NEJMoa1407222 . Chimeric antigen receptor T cells for sustained remissions in leukemia. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH , Porter DL, Grupp SA.
- ↑ Cancer Immunol Immunother. 2014 Oct; 63 (10): 1061-71. doi: 10.1007 / s00262-014-1575-2 . Epub 2014 Jul 4. A phase I clinical trial combining dendritic cell vaccination with adoptive T cell transfer in patients with stage IV melanoma. Poschke I, Lövgren T, Adamson L, Nyström M, Andersson E, Hansson J, Tell R, Masucci GV, Kiessling R.
- ↑ Anticancer Res. 2013 Dec; 33 (12): 5495-500. Wilms' Tumor Gene 1 (WT1) - loaded dendritic cell immunotherapy in patients with uterine tumors: a phase I / II clinical trial. Coosemans A, Vanderstraeten A, Tuyaerts S, Verschuere T, Moerman P, Berneman ZN, Vergote I, Amant F, VAN Gool SW.
- ^ Immunotherapy. 2012 Jul; 4 (7): 703-18. doi: 10.2217 / imt.12.40 . Dendritic cell engineering for tumor immunotherapy: from biology to clinical translation. Bhargava A, Mishra D, Banerjee S, Mishra PK.
- ^ PA Bradbury and FA Shepherd: Immunotherapy for lung cancer. In: J Thorac Oncol 3, 2008, pp. 164-170. PMID 18520304 (Review)
- ^ B. Ebbell: The Papyrus Ebers: the greatest Egyptian medical document. Oxford University Press, 1937.
- ↑ a b c S. A. Hoption Cann u. a .: Dr William Coley and tumor regression: a place in history or in the future. In: Postgraduate Medical Journal 79, 2003, pp. 672-680. PMID 14707241 (Review)
- ↑ TF Murphy: Case studies in biomedical research ethics. MIT Press, 2004 ISBN 0-262-63286-1 , p. 112.
- ↑ a b C. V. Ichim: Revisiting immunosurveillance and immunostimulation: Implications for cancer immunotherapy. In: J Transl Med 3, 2005, 8. PMID 15698481 (Review in Open Access )
- ^ P. Bruns: The healing effect of the erysipelas on tumors. In: Beitr Klin Chir 3, 1888, pp. 443-466.
- ↑ P. Klärner: Regressions of Yoshida sarcoma after a single injection of a lipopolysaccharide from E. Coli. In: Zeitschrift für Krebsforschung 62, 1958, pp. 291-296.
- ^ WB Coley: II. Contribution to the Knowledge of Sarcoma. In: Ann Surg 14, 1891, pp. 199-220. PMID 17859590 , PMC 1428624 (free full text)
- ^ DB Levine: The Hospital for the Ruptured and Crippled: William Bradley Coley, Third Surgeon-in-Chief 1925-1933. In: HSS J 4, 2008, pp. 1-9. doi: 10.1007 / s11420-007-9063-2 PMID 18751855 PMC 2504278 (free full text)
- ^ DB Levine: The Hospital for the Ruptured and Crippled: Knight to Gibney. 1870-1887. In: HSS J 2, 2006, pp. 1-6.
- ^ WB Coley: Contribution to the knowledge of sarcoma. In: Ann Surg 14, 1891, pp. 199-220.
- ^ WB Coley: The Treatment of Malignant Tumors by Repeated Innoculations of Erysipelas: With a Report of Ten Original Cases. In: American Journal of the Medical Sciences 10, 1893, pp. 487-511.
- ↑ B. Wiemann and CO Starnes: Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. In: Pharmacol Ther 64, 1994, pp. 529-564. PMID 7724661
- ↑ WB Coley: End results in Hodgkin's disease and lymphosarcom treated by the mixed toxins of erysipelas nad bacillus prodigiosus, alon or combined with radiation. In: Ann Surg 88, 1928, pp. 641-667. PMID 17865976
- ^ A b E. F. McCarthy: The Toxins of William B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. In: Iowa Orthop J 26, 2006, pp. 154-158. PMID 16789469 PMC 1888599 (free full text)
- ↑ J. Bickels et al. a .: Coley's toxin: historical perspective. ( Memento of the original from February 1, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. In: Isr Med Assc J 4, 2002, pp. 471-472. PMID 12073431
- ^ PN Leech: Erysipelas and Prodigiosus toxins (Coley). In: JAMA 103, 1934, pp. 1067-1069.
- ^ A. Pollack: A Revival for Immunity. In: New York Times October 5, 2005 issue
- ↑ P. Ehrlich: About the current status of carcinoma research. In: Ned Tijdschr Geneeskd 5, 1909, pp. 273-290.
- ↑ HF von Doerr u. a .: Pathology of the thymus. Verlag Springer, 1998, ISBN 3-540-64065-7 . P. 103.
- ^ R. Pearl: Cancer and tuberculosis. In: Am J Hygiene 9, 1929, p. 97
- ↑ L. Thomas: Cellular and Humoral Aspects of Hypersensitivity. HS Lawrence (Editor), Hoeber-Harper, 1959.
- ^ FM Burnet: The concept of immunological surveillance. In: Prog Exp Tumor Res 13, 1970, pp. 1-27. PMID 4921480
- ↑ E. Coeugniet: Editorial. In: Onkologie June 1989, pp. 3-4.
- ↑ S. Höpner: Characterization of an hABL-specific CD4 + T cell response and the use of AdEtOH as a catalyst for peptide loading. Dissertation, FU Berlin, 2008
- ↑ J. Rygaard and CO Povlsen: The mouse mutant nude does not develop spontaneous tumors. An argument against immunological surveillance. In: Acta Pathol Microbiol Scand [B] Microbiol Immunol 82, 1974, pp. 99-106. PMID 4597815
- ^ O. Stutman: Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. In: Science 183, 1974, pp. 534-536. PMID 4588620
- ↑ JR Maleckar LA Sherman: The composition of the T cell receptor repertoire in nude mice. In: J Immunol 138, 1987, pp. 3873-3876. PMID 2953792
- ↑ S. Ikehara et al. a .: Functional T cells in athymic nude mice. In: PNAS 81, 1984, pp. 886-888. PMID 6608104
- ↑ RB Herberman and HT Holden: Natural cell-mediated immunity. In: Adv Cancer Res 27, 1978, pp. 305-377. PMID 356546 (Review)
- ↑ C. Huber (editor) a. a .: Cancer immunotherapies. Deutscher Ärzteverlag, 2007, ISBN 3-7691-1212-1 , p. 5.
- ↑ CY Li et al. a .: Cytokines and immunogenic therapy for solid tumors. (PDF; 746 kB) In: Cell Mol Immunol 2, 2005, pp. 81-91. PMID 16191413 (Review)
- ↑ James P. Allison and Carl H. June receive the Paul Ehrlich and Ludwig Darmstaedter Prize 2015 , press release of the Paul Ehrlich Foundation of January 29, 2015, accessed on February 6, 2015