HPV vaccine
HPV vaccines protect against certain types of sexually transmitted human papillomavirus (HPV) by building up immunity and thus serve to prevent cancer . The World Health Organization recommends that all countries include HPV vaccination in their national immunization programs, the HPV vaccine is on the World Health Organization's list of Essential Medicines . The high-risk HPV types 16 and 18 are responsible for around 70% of all cervical cancer cases worldwide . According to the Robert Koch InstituteIn 2014, 1,506 women died of cervical cancer in Germany. The papillomaviruses type 6 and 11 are primarily responsible for the development of genital warts (genital warts). Three HPV vaccines are currently approved: a double vaccine that is only effective against HPV types 16 and 18, a quadruple vaccine that is directed against HPV types 6, 11, 16 and 18, and a nine- fold vaccine - Vaccine that protects against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. The effectiveness of the quadruple vaccine in preventing the precursors of cervical cancer caused by the corresponding HPV types was 98 to 100% in clinical studies for subjects who were not previously infected with HPV.
The vaccines are only preventive ( prophylactic ). It cannot treat existing infections. According to the current state of knowledge, HPV vaccines are well tolerated and safe. The most common adverse effects are local reactions such as redness, swelling and pain at the injection site. The preventive medical check-up for early detection of cervical cancer ( Pap test ) is still recommended, as not all cancerogenic HPV types are covered by the vaccination.
Risks of HPV infection
The high-risk HPV types 16 and 18 are held responsible for around 70% of all cervical cancer cases in women worldwide. 55.3% of all cervical cancer precursors of severity CIN 2 and 3 were associated with HPV 16 in two German studies, 6.4% with HPV 18, HPV 45 in 8.5% and HPV 31 in 6.4% of the cases . High-risk virus subsets are not only implicated in causing cervical cancer; they are also found in cancers of the penis , vulva , anus and mouth . In 2005, the WHO classified HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 66 as carcinogenic.
The so-called low-risk HPV types 6 and 11 are responsible for the development of over 90% of genital warts (genital warts), which are the most common viral sexually transmitted disease worldwide . It is estimated that around 1% of the European and German population in the 15 to 49 age group is affected by these benign tumors. Studies in recent years show an increasing frequency. Genital warts often lead to anxiety and psychosocial complications as well as partner problems and thus to a significant reduction in quality of life .
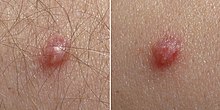
Pronounced genital warts on the penis
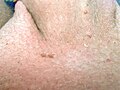
Pronounced genital wart infestation in the vulva
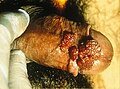
Genital wart infestation of the anus
development
A quadruple vaccine manufactured by Sanofi Pasteur MSD was first approved in the USA in June 2006 by the Food and Drug Administration and in September 2006 by the EU Commission following a central approval procedure in the countries of the European Union . In November 2006, it was approved by Swissmedic in Switzerland. In Europe the trade name is Gardasil or Silgard . The vaccine contains purified, recombinantly produced L1 proteins from the capsid of the four papilloma virus types 6, 11, 16 and 18, which spontaneously assemble to form virus-like particles (VLP). According to the European Medicines Agency, 1.5 million patients in Europe had been vaccinated with Gardasil by January 2008 . This was followed by extensions of indications for the prevention of preliminary stages of vaginal and vulvar carcinoma ( USA : September 2008, EU: July 2008) as well as for the prevention of genital warts (USA: in October 2009 extension to men and boys, EU: November 2011) and Prevention of preliminary stages of malignant lesions in the anal area and anal cancer (USA: December 2010, EU: June 2014).
The bivalent vaccine developed by GlaxoSmithKline and sold under the trade name Cervarix was approved in Australia in May 2007 and in the European Union on September 20, 2007 . FDA approval was granted in October 2009. This vaccine also contains recombinant L1 proteins from the capsid in VLPs, but only of papillomavirus types 16 and 18.
In a Phase III study by MSD of a new recombinant nonavalent vaccine against virus types 6, 11, 16, 18, 31, 33, 45, 52, and 58 called V503, vaccination prevented 97% of high-grade vaginal, vulvar and cervical cancer precursors caused by these types of viruses. The new vaccine produced the same or better immune response than Gardasil in HPV types 6, 11, 16 and 18. V503 was approved in the USA in December 2014 and in the EU in June 2015 under the trade name Gardasil 9 , which in Germany has been available since 2016.
An international working group reported in June 2013 on the development of a multivalent vaccine that could protect in the future not only against the majority of genital high- and low-risk types of the virus, but also against those guys, for the formation of skin warts are responsible . An evaluation in clinical studies is still pending.
effectiveness
As a general rule
In 2018, a Cochrane review rated the HPV vaccination as effective and safe. Scientists from Scotland shared this view. The evaluation of over 100,000 health data has shown that routine HPV vaccinations in girls aged 12-13 years have significantly reduced the likelihood of abnormal cells and cervical intraepithelial neoplasia (CIN; precursors of cervical cancer). The earlier an HPV vaccination took place, the more effective the HPV vaccination was.
A large meta-analysis of over 60 million patient data and an eight-year follow-up has shown that the HPV vaccination has a significant influence on the decline in HPV diseases and the CIN2 + cervical cancer precursor. For example, CIN2 + decreased by 51% in women between 15 and 19 years of age and by 31% in women between 20 and 24 years of age 5-9 years after the HPV vaccination.
In the USA it was observed that after the introduction of the quadrivalent vaccine, the incidence of the corresponding vaccinated, carcinogenic HPV strains decreased significantly compared to the pre-vaccination period: in 14 to 19 year olds from 11.5% to 1.8% and in 20 - up to 24 year olds from 18.5% to 5.3%. In Great Britain, 10 years after the introduction of HPV vaccination, it was found that 16-18 year old women were no longer infected with the two high-risk strains 16 and 18.
Studies on the effectiveness of Gardasil
The effectiveness of the tetravalent vaccine ( Gardasil ) was investigated in four placebo-controlled , randomized and double-blind phase II and phase III studies . In women who were not infected with the corresponding human papillomavirus at the time of vaccination, the vaccination prevented infection in almost all women (96-100%). In the vaccinated study participants, the vaccination prevented the occurrence of cervical intraepithelial neoplasms, a surrogate marker for cervical cancer caused by the corresponding HPV types, in 98–100% of the test subjects. For example, in the so-called Future II study, the occurrence of CIN grade 2 or more severe precursors of cervical cancer was registered. While there was one case of CIN in the group of women treated with the vaccine (5305 women in total ), there were 42 cases in the group of women treated with placebo (5260 women). In addition, the vaccination protected 98-100% of the study participants from developing anogenital warts. For ethical reasons, the independent Data Safety Monitoring Board recommended vaccination of the subjects treated with placebo as soon as possible .
If women with HPV infections existing at the start of the study are included in the analysis and also those who received less than the three required vaccine doses, the effectiveness of Gardasil against HPV-caused precursors of cervical cancer is lower, but it does exist. In the combined interim analysis of the four relevant efficacy studies carried out for approval, the efficacy against CIN 2/3 / AIS caused by HPV types 16 and / or 18 was 39%.
Boys 9 to 15 years of age developed immunity after vaccination . The quadrivalent vaccine was judged to be effective in a study of 4,065 boys and men aged 16 to 26 years. A worldwide clinical study for the prevention of venereal diseases, especially genital warts, is ongoing for homosexual men, as well as studies with the HPV vaccine. The aim is a vaccination to prevent anal and penile cancer , the development of which is also associated with infections with HPV.
In 2011, the first data were published after the introduction of a comprehensive vaccination program with a quadrivalent vaccine. The program was established in Australia in 2007. The vaccination rate there is 80 to 90 percent. Three years after the start of the vaccination campaign, the precancerous stages in need of treatment in girls under 18 years of age were 59 percent lower than before.
Studies on the effectiveness of Cervarix
The bivalent vaccine Cervarix protects against the cancer-causing HPV types 16 and 18. Statistically significant efficacy of this drug could only be demonstrated for HPV 16 in a large study. Clinical data for Cervarix are currently available over a period of 5.5 years. According to new data, Cervarix also protects against infections with HPV types that are not in the vaccine. This so-called cross protection extends to different degrees to virus types 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59. Virus types 31 and 45 (in addition to HPV 16 and 18) in particular have a high oncogenic potential . Cross protection with the phylogenetically related HPV types 45, 52 and 58 is also known from Gardasil . In a study published in PLoS ONE, it was shown that Cervarix also protects against oral infections. This is particularly relevant because of the increasing tumors in the head and neck area, which are increasingly triggered by HP viruses.
According to data from the PATRICIA study ( Papilloma Trial against Cancer in Young Adults ), a phase III study (HPV 008) with Cervarix , in which 18,644 women in 14 countries in North and South America, Europe and the Asia-Pacific region were involved Aged between 15 and 25 years of age, the CIN 2+ findings were reduced by 70% (33 versus 110 cases) and the CIN 3+ findings by 87% (3 versus 23 cases). The rate of conizations decreased by 68.8 percent (i.e. to 31.2%).
Comparison of Gardasil and Cervarix
The first direct comparative study between the two preparations (HPV 010), which included 1,106 healthy women between 18 and 45 years of age from 40 US centers, showed a significantly higher immune response from the bivalent vaccine Cervarix , which is most likely explained by a new adjuvant ( AS04 ) can. It is unclear whether this increased immune response is relevant for protection or its duration.
In November 2008, 13 scientists from various German research institutions demanded a reassessment of the HPV vaccination on the grounds that the effectiveness could be significantly lower than assumed. In this reassessment by the Robert Koch Institute on August 10, 2009, vaccination was still recommended. Information media that give the impression that an HPV vaccination provides 100% protection against cervical cancer were classified as dubious according to the current state of knowledge.
execution
The vaccination schedule depends on the age of the person to be immunized: from 14 years of age ( Gardasil ) or from 15 years of age ( Cervarix ), the basic immunization is carried out using three intramuscular injections , each two and six months after the first injection ( Gardasil ) or after one and six months ( Cervarix ). Children aged nine to 13 years ( Gardasil ) or up to 14 years ( Cervarix ) are vaccinated according to a two-dose scheme with just one additional injection six months after the first injection. Whether a booster vaccination will be necessary is still the subject of further investigations. However, follow-up observations have so far not given any indications of a decrease in vaccination protection, although the informative value of the studies for long-term protection beyond 5 years was worse than for short-term protection up to 5 years. Data from two large phase III studies with the bivalent HPV vaccine Cervarix indicate that a single vaccination is apparently sufficient.
Vaccination recommendation
The vaccination has entered the vaccination program of over 80 countries such as Australia , Canada , New Zealand and South Korea worldwide . In the United States , vaccination has become a legal requirement in many states.
| country | Date of vaccination recommendation |
|---|---|
| Belgium | May 11, 2007 |
| Denmark | 4th October 2007 |
| Germany | March 26, 2007 |
| France | March 16, 2007 |
| Greece | February 7, 2008 |
| Ireland | August 5, 2008 |
| Iceland | February 19, 2008 |
| Italy | February 28, 2007 |
| Luxembourg | February 27, 2007 |
| Netherlands | March 31, 2008 |
| Norway | April 12, 2007 |
| Austria | December 20, 2006 |
| Portugal | December 10, 2007 |
| Sweden | February 26, 2008 |
| Switzerland | June 18, 2007 |
| Spain | October 10, 2007 |
| United Kingdom | October 26, 2007 |
In Japan, the vaccination recommendation was suspended after reports of several suspected cases of serious side effects ( complex regional pain syndrome ). The German Federal Institute for Vaccines and Biomedical Medicines ( Paul Ehrlich Institute ) did not see any risk signals for the occurrence of this syndrome in 2013 after evaluating all reported suspected cases and the literature. The European Medicines Agency agreed with this assessment after a review in November 2015. In 2017, members of 17 Japanese specialist societies called for the vaccination recommendation to be renewed.
Germany
The Standing Vaccination Commission (STIKO) recommended HPV vaccination for girls from March 2007.
After the introduction of the HPV vaccination, a discussion broke out in Germany about its costs, cost efficiency, advertising measures by the manufacturers and the risks of vaccination. In November 2008, 13 health scientists published a “manifest” on a website of Bielefeld University in which they called for an end to “misleading information” on the effectiveness of the HPV vaccination and a renewed review of the STIKO recommendation. In particular, it was criticized that no data on protection against cervical cancer were available and that the STIKO had decided on the HPV vaccination recommendation before the decisive study data were published. The professional association of gynecologists (BVF), the German Society for Gynecology and Obstetrics (DGGG), the Society for Virology (GfV) and the German Association for Combating Virus Diseases (DVV) as well as the Nobel Prize winner Harald zur Hausen rejected the criticism. In a statement on the vaccination in 2009, the STIKO referred to the criticism and, after evaluating all available scientific data, came to the conclusion that the vaccination is still recommended for girls aged 12 to 17 years. Data from nationwide programs with high vaccination rates, such as in Australia, confirm the STIKO's recommendation.
In 2014, the STIKO lowered the age limit for girls in its recommendations. Since summer 2014 she has been recommending vaccination against HPV types 16 and 18 for all girls between the ages of 9 and 14 using a 2-dose scheme. Thereafter, missed vaccinations against HPV by the age of 18 should be made up with a total of 3 vaccine doses. The full series of vaccinations should be completed before the first sexual intercourse.
In 2018, the German Society for Urology (DGU) and the Professional Association of German Urologists (BDU) also rated the vaccination as recommended for boys.
On June 5, 2018, the STIKO passed a resolution recommending the HPV vaccination of boys aged 9 to 14 years. In the event that this period is not observed, a catch-up vaccination is recommended up to the age of 17 years. The recommendation will formally come into force with its publication and the scientific justification in Epidemiological Bulletin 26/2018 on June 28, 2018.
Austria
Since 2007, the Supreme Sanitary Council has recommended vaccination against oncogenic HPV types for all 9 to 17 year old girls and women, if possible before their first sexual contact. The vaccination of boys or male adolescents is described in the Austrian vaccination plan 2009 as "in principle sensible" in order to break the chain of infection. When using a vaccine that also protects against condyloma- causing viruses, the male adolescents would also have a personal advantage.
Switzerland
The Federal Office of Public Health (FOPH) and the Federal Commission for Vaccination (EKIF) recommend vaccinating all girls between the ages of 11 and 14 against human papillomavirus with two doses of vaccination every 4 to 6 months. A catch-up vaccination in the three-dose scheme (0 / 1-2 / 6 months) is recommended for adolescent girls up to their 20th birthday. Afterwards, young women between 20 and 26 years of age are offered the HPV vaccine as a supplementary vaccination based on individual recommendations. Vaccination of boys and young men between the ages of 11 and 26 has also been recommended in Switzerland since March 2015.
costs
With the exception of Finland, all Western European countries have so far decided to finance the HPV vaccination. International studies show a cost-benefit analysis of vaccination assuming a lifelong period of vaccination protection, a cost-effectiveness ratio that is below the threshold of 50,000 euros per additional quality-adjusted year of life (QALY), a year of life with a good quality of life. As a result, most studies conclude that HPV vaccination is cost-effective. However, an assessment can currently only be made using model calculations, the result of which depends on the factors considered (such as assumptions about the unknown effect against cervical cancer and unknown duration of vaccination protection) and the framework conditions in the respective country. In the Netherlands, for example, it was concluded that vaccination is not cost-effective. Vaccinating women 35 years of age and older is not cost effective in the US. Nobel laureate Harald zur Hausen himself “… pointed out at every opportunity that the vaccine is currently too expensive. This applies in particular to developing countries, in which cervical cancer is in part the most common cancer in women. ”In Kenya , the vaccine costs around half the average annual income.
Germany
The cost of the vaccine is around 150 euros per required single dose. In 2008, the health insurance companies in Germany spent 244 million euros on the two vaccines Gardasil and Cervarix , according to the figures from the 2009 Drug Prescription Report . After the vaccination has been included in the guideline of the Federal Joint Committee on protective vaccinations according to Section 20d (1) SGB V , the statutory health insurance companies will cover the vaccination costs for girls between the ages of 9 and 17. Some health insurance companies cover the cost of vaccinating women up to the age of 26 as a voluntary statutory benefit.
The Standing Vaccination Commission (STIKO) has recommended vaccination for 9 to 14-year-old boys since 2018 , the costs are covered by all statutory health insurances up to the age of 17, some health insurances assume it as a voluntary statutory benefit up to the age of 26.
Austria
The HPV vaccination for girls and boys was included in the school vaccination program on August 12, 2013 and has therefore been free of charge for children aged 9 to 12 since February 2014. Discounted prices apply to young people up to 15 years of age.
The vaccination could already be obtained at reduced prices in individual federal states as part of school or public vaccination campaigns. In Lower Austria , for example, a vaccination campaign started in 2007, in which all girls and women between 9 and 26 years of age could be vaccinated at the Lower Austrian regional clinics at a discounted price of 90 euros per dose (instead of 208 euros). In Burgenland , the vaccination was offered at a reduced price to all girls in the 5th to 8th grade from September 2008 through a school vaccination campaign. The promotional price including state funding was 90 euros (instead of 208 euros) per vaccination. Girls and women between the ages of 15 and 26 and boys between the ages of 9 and 15 were also able to obtain the vaccine at a reduced price through a public vaccination campaign in Burgenland. Here the promotional price was 150 euros (instead of 208 euros) per vaccination.
In 2007, the Ludwig Boltzmann Institute saw the vaccination recommendation only as a third option due to the high level of uncertainty and high costs, and thus unclear cost effectiveness.
Switzerland
For female and male adolescents aged 11 to 26, the costs of the HPV vaccination are covered by the mandatory health insurance . The prerequisite is that the service is provided as part of a cantonal vaccination program. The bivalent (HPV type 16/18) and quadrivalent (HPV type 6/11/16/18) HPV vaccination is reimbursed to the canton by insurance companies at a price of CHF 91.50 per dose. The total flat rate reimbursement for health insurers is 159 francs per vaccination. From July 2016 this will also be granted to boys and young men in the same age group.
unwanted effects
At the time of approval, data from several clinical studies with over 20,000 participants were available on the tolerability of the HPV vaccination. Since Gardasil was approved , several million girls and women around the world have been vaccinated. The occurrence of adverse effects , which may be caused by the HPV vaccination, is recorded in special monitoring programs.
On the basis of the available study data, the HPV vaccination is included in the German Health Technology Assessment, the US regulatory authority for drugs (FDA), the Centers for Disease Control and Prevention (CDC), the European Medicines Agency (EMA), the Fédération Internationale de Gynécologie et d'Obstétrique (FIGO) and the Global Advisory Committee of the World Health Organization (WHO) have been rated as safe and well tolerated.
The most common undesirable effects observed in the controlled studies were local reactions (redness, swelling and pain) at the injection site with both vaccines. In the Gardasil studies , local reactions occurred in around 83% of women in the vaccine group and 73% of women in the placebo group. In almost all cases, the study participants received a mixture of water and the adjuvant amorphous aluminum hydroxyphosphate sulfate as an (active) placebo . The most common systemic reactions were headache, fatigue, muscle pain, and nausea; they occurred with the same frequency in the vaccine and placebo groups. Fever was recorded in 10-15% of women in both the vaccine and placebo groups. Serious adverse effects also occurred equally frequently in the vaccination and placebo groups and consisted of narrowing of the airways ( bronchospasm ), gastroenteritis , increase in blood pressure, severe headache, pain at the injection site and reduced mobility in the adjacent joint. A study that looked at the incidence of chronic illness up to four years after receiving the HPV vaccine or placebo found that 3% of women in the vaccine group had a chronic illness. In the placebo group, the proportion of new chronic diseases was 5%. With regard to the number of deaths that have occurred, a meta-analysis of the clinical studies showed that deaths were equally common in the vaccine and placebo groups and were mostly caused by accidents. Vaccination was not considered the cause of any of the deaths.
Since the US approval of the HPV vaccine Gardasil in June 2006, the FDA and the Centers for Disease Control and Prevention had received 17,160 reports of potential adverse drug reactions from approximately 26 million vaccine doses through September 2009 through surveillance programs. The majority of the undesirable effects (92%) were classified as not serious and consisted of local reactions, headache, nausea, fever and syncope . 8% of the reported adverse effects were serious. Serious cases were defined as those that resulted in hospital admission, life-threatening illness, irreversible disability or death. For example, diseases of Guillain-Barré syndrome , thromboses and deaths were reported in connection with the vaccination . All serious cases were investigated for possible causation by Gardasil ; in no case could a causal connection between the vaccination and the respective disease be proven. The suspected increased risk of thrombosis could not be confirmed by a Danish case-control study. Another large cohort study from Scandinavia was able to show that the HPV vaccination is not associated with an increased risk of multiple sclerosis (MS) and other demyelinating diseases .
Cervarix had a spectrum of side effects similar to Gardasil : the most common side effects observed after administration were pain at the injection site (78%) and headache and muscle pain . For both vaccines, swelling of the lymph nodes ( lymphadenopathy ) and anaphylactoid reactions have been reported in isolated cases . An infertility by ovarian failure is considered another very rare adverse event reported.
In November 2015, the European Medicines Agency (EMA) announced that it had investigated HPV vaccines in a review that did not question the benefit-risk ratio, but instead examined whether two undesirable effects rarely reported in connection with the vaccination Effects, complex regional pain syndrome ( CRPS ) and postural orthostatic tachycardia syndrome (POTS), occur more frequently in vaccinated women than in unvaccinated women. The evaluation of all available scientific information and reports with regard to the two syndromes showed that the reporting rates of these diseases observed in the temporal connection with HPV vaccinations correspond to the expected frequency of occurrence in the age group examined (female adolescents aged 10 to 17 years) and thus there is no indication of a connection. Following a criticism of this review by the Danish scientist Louise Brinth published in December 2015 , the director of the Nordic Cochrane Center, Peter C. Gøtzsche , filed a formal complaint about the official assessment report of the EMA in May 2016.
In 2018, a Cochrane review rated the vaccination as effective and safe. This review was also criticized by Gøtzsche and his colleagues. He was expelled from the Cochrane Collaboration on September 26, 2018 for "continuing bad behavior that is inconsistent with the principles and governance of Cochrane".
pregnancy and breast feeding period
There is limited data from clinical studies regarding the safety of vaccination with HPV vaccines during pregnancy. However, these did not show an increased risk for the embryo or the fetus . For example, in almost 1,800 women who were unintentionally vaccinated during pregnancy, no evidence of a negative outcome was observed. There is also no evidence of adverse effects of the vaccination during breastfeeding. Although neither animal experiments nor the available data from clinical studies in humans give any indications of direct or indirect harmful effects, the STIKO does not currently classify vaccination during pregnancy as “safe” and the manufacturers advise against its use during pregnancy. Gardasil has been tested against placebo in clinical studies in breastfeeding mothers. The frequency of side effects in mother and infant was similar in both groups. The immunogenicity of the vaccine in nursing mothers was similar to that of the vaccine in women who were not breastfeeding at the time of vaccination. Gardasil can therefore be given to women who are breastfeeding. Cervarix should only be used during breast-feeding if the potential benefits outweigh the potential risks, as the effects on breast-fed infants whose mothers received Cervarix have not been studied in clinical studies.
The authors of the S3 guideline on vaccination prevention of HPV-associated neoplasms of the Paul Ehrlich Society for Chemotherapy (AG HPV Management Forum), the German STD Society and the German Dermatological Society generally consider vaccination during breastfeeding to be justifiable.
Therapeutic HPV vaccines
In addition to the preventive vaccines, such as B. Gardasil and Cervarix, a therapeutic HPV vaccine under the name VGX-3100, MEL-1 or MVA-E2 is being developed for those who are already infected. This potential vaccine is currently in phase 3 of clinical development, i.e. not yet approved . The study is expected to be completed in August 2020.
Scientists at the German Cancer Research Center developed a therapeutic vaccine that could be used to combat cervical cancer caused by HPV in animal experiments on mice. The tumors regressed in half of the vaccinated animals.
literature
- Ulrich R. Hengge (Ed.): HPV Infection and Cancers of the Skin - Diagnostics and Therapy. Uni-Med Science, Bremen 2008, ISBN 978-3-8374-1076-1 .
- Gregory D. Zimet, Marcia L. Shew, Jessica A. Kahn: Appropriate use of cervical cancer vaccine . In: Annual Review of Medicine . tape 59 , 2008, p. 223-236 , doi : 10.1146 / annurev.med.59.092806.131644 , PMID 18186704 .
Web links
- S3 guidelines for vaccination prevention of HPV-associated neoplasms of the Paul Ehrlich Society for Chemotherapy (AG HPV Management Forum), the German STD Society and the German Dermatological Society. In: AWMF online (as of 12/2013)
- Information from the Robert Koch Institute on HPV vaccines
- European Medicines Agency publications on Gardasil and Cervarix
- HPV vaccination: answers to common questions - What does vaccination protect against? Cancer information service of the German Cancer Research Center (DKFZ), Heidelberg, May 8, 2014; Retrieved September 4, 2014.
- EMA - overview of approved vaccines
Individual evidence
- ^ WHO Model Lists of Essential Medicines. (PDF) In: WHO. 2019, accessed April 4, 2020 .
- ↑ Human papillomavirus vaccines: WHO position paper, May 2017 . In: WHO (Ed.): Weekly epidemiological record . tape 92 , no. 19 , 2017, p. 241-268 , PMID 28530369 ( who.int [PDF]).
- ↑ Cancer information service, German Cancer Research Center: HPV vaccination .
- ^ Cervical cancer at the Center for Cancer Registry Data (ZfKD) at the Robert Koch Institute , as of February 28, 2018
- ↑ Maura L. Gillison, Anil K. Chaturvedi, Douglas R. Lowy: HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. In: Cancer 113, 2008, pp. 3036-3046, doi: 10.1002 / cncr.23764 , PMID 18980286 , online (PDF; 177 kB).
- ↑ Stefanie J. Klug, Meike Hukelmann, Bettina Hollwitz, Nurgül Düzenli, Betti Schopp, Karl-Ulrich Petry, Thomas Iftner: Prevalence of human papillomavirus types in women screened by cytology in Germany. In: J Med Virol 79 (2007), pp. 616-25, PMID 17385693 .
- ^ V. Cogliano, R. Baan, K. Straif, Y. Grosse, B. Secretan, F. El Ghissassi: Carcinogenicity of human papillomaviruses. In: Lancet Oncol , 6, 2005, p. 204, PMID 15830458 .
- ↑ a b S3 guideline for vaccination prevention of HPV-associated neoplasms of the Paul Ehrlich Society for Chemotherapy (AG HPV Management Forum), the German STD Society and the German Dermatological Society. In: AWMF online (as of 06/2008)
- ↑ For information on the approval of Gardasil, see: Approval of a new vaccine: Gardasil . In: Swissmedic Journal . 6th year, No. 1, 2007, ISSN 0026-9212 , pp. 12-13 ( PDF file; 522.1 kB ).
- ↑ a b European Medicines Agency : Gardasil - European Public Assessment Report. accessed on February 9, 2016.
- ↑ Press release on the safety of Gardasil. (PDF; 28 kB) European Medicines Agency, January 24, 2008
- ^ A b Center for Biologics Evaluation and Research: Approved Products - Gardasil .
- ↑ a b c Technical information Gardasil (PDF)
- ↑ FDA Approves New Indication for Gardasil to Prevent Genital Warts in Men and Boys. ( Memento of May 10, 2015 in the Internet Archive ), FDA press release of October 16, 2009.
- ↑ FDA: Gardasil approved to prevent anal cancer ( Memento of August 7, 2015 in the Internet Archive ), FDA press release of December 22, 2010
- ↑ Gardasil Procedural steps taken and scientific information after the authorization (PDF), on ema.europa.eu
- ↑ FDA approval of Cervarix .
- ↑ a b European Medicines Agency: Cervarix - European Public Assessment Report. Retrieved February 14, 2009.
- ↑ Broad Spectrum HPV (Human Papillomavirus) Vaccine Study in 16-to 26-Year-Old Women (V503-001) on ClinicalTrials.gov
- Jump up ↑ Warner K Huh, Elmar A Joura, Anna R Giuliano, Ole-Erik Iversen, Rosires Pereira de Andrade, Kevin A Ault, Deborah Bartholomew, Ramon M Cestero, Edison N Fedrizzi, Angelica L Hirschberg, Marie-Hélène Mayrand, Angela Maria Ruiz -Sternberg, Jack T Stapleton, Dorothy J Wiley, Alex Ferenczy, Robert Kurman, Brigitte M Ronnett, Mark H Stoler, Jack Cuzick, Suzanne M Garland, Susanne K Kjaer, Oliver M Bautista, Richard Haupt, Erin Moeller, Michael Ritter, Christine C Roberts, Christine Shields, Alain Luxembourg: Final efficacy, immunogenicity, and safety analyzes of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomized, double-blind trial. Lancet 390 (2017), pp. 2143-2159, PMID 28886907 , DOI: 10.1016 / S0140-6736 (17) 31821-4
- ↑ FDA approves Gardasil 9 for prevention of certain cancers caused by five additional types of HPV FDA News Release.
- ↑ European Medicines Agency - Find medicine - Gardasil 9 .
- ↑ a b HPV vaccination - preventing cervical cancer .
- ↑ Christina Schellenbacher, Kihyuck Kwak, Dieter Fink, Saeed Shafti-Keramat, Bettina Huber, Christoph Jindra, Helena Faust, Joakim Dillner, Richard BS Roden, Reinhard Kirnbauer: Efficacy of RG1-VLP Vaccination against Infections with Genital and Cutaneous Human Papillomaviruses. In: Journal of Investigative Dermatology , 2013; 133: 2706-2713, PMID 23752042 , doi: 10.1038 / jid.2013.253
- ↑ New HPV vaccines could offer broader protection. Communication from the Medical University of Vienna dated February 26, 2014.
- ↑ M. Arbyn et al .: Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No .: CD009069. DOI: 10.1002 / 14651858.CD009069.pub3 .
- ^ Deutscher Ärzteverlag GmbH, editorial office of the Deutsches Ärzteblatt: The HPV vaccination works. April 11, 2019, accessed May 27, 2019 .
- ↑ Tim Palmer et al .: Prevalence of cervical disease at age 20 after immunization with bivalent HPV vaccine at age 12-13 in Scotland: retrospective population study . In: BMJ . tape 365 , April 3, 2019, p. l1161 , doi : 10.1136 / bmj.l1161 , PMID 30944092 .
- ↑ Mélanie Drolet et al .: Population-level impact and herd effects following the introduction of human papillomavirus vaccination programs: updated systematic review and meta-analysis . In: Lancet (London, England) . tape 394 , no. 10197 , August 10, 2019, p. 497-509 , doi : 10.1016 / S0140-6736 (19) 30298-3 , PMID 31255301 .
- ^ Nancy M. McClung et al .: Declines in Vaccine-Type Human Papillomavirus Prevalence in Females Across Racial / Ethnic Groups: Data From a National Survey . In: Journal of Adolescent Health . tape 65 , no. 6 , December 1, 2019, p. 715-722 , doi : 10.1016 / j.jadohealth.2019.07.003 .
- ^ HPV prevalence in sexually active young females in England. Public Health England, January 22, 2020, accessed January 28, 2020 .
- ↑ Diane M. Harper, Martin S. Hirsch, Barbara H. McGovern: Human papillomavirus vaccines. In: DS Basow (Ed.): UpToDate. UpToDate, Waltham, MA, 2008.
- ↑ a b L Rambout, L. Hopkins, B. Hutton, D. Fergusson: Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. In: CMAJ. 2007 Aug 28; 177 (5): 469-79. PMID 17671238
- ^ European Public assessment report - Scientific discussion (PDF) of October 5, 2006 (English).
- ^ Anna R. Giuliano et al .: Efficacy of Quadrivalent HPV Vaccine against HPV Infection and Disease in Males . In: New England Journal of Medicine . tape 364 , no. 5 , February 3, 2011, p. 401-411 , doi : 10.1056 / NEJMoa0909537 , PMID 21288094 , PMC 3495065 (free full text).
- ↑ Rüdiger Meyer: Early regression of cervical lesions through HPV vaccination. German Ärzteblatt 108 (2011), p. A-1458, online (PDF document)
- ↑ Julia ML Brotherton, Masha Fridman, Cathryn L May, Genevieve Chappell, A Marion Saville, Dorota M Gertig: Early effect of the HPV vaccination program on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 377 (2011), 2085-2092, doi: 10.1016 / S0140-6736 (11) 60551-5
- ↑ a b Paavonen J et al .: Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomized controlled trial . In: The Lancet . 369, No. 9580, June 2007, pp. 2161-70. doi : 10.1016 / S0140-6736 (07) 60946-5 . PMID 17602732 .
- ↑ a b c Vera Zylka-Menhorn: HPV vaccination: The study world has been expanded. Dtsch Arztebl 106 (2009), A-1185.
- ↑ a b c O Damm, M Nocon, S Roll, C Vauth, SN Willich, W Greiner: Vaccination against human papillomavirus (HPV) for the prevention of HPV 16/18 induced cervical carcinomas and their precursors. Health Technology Assessment series, 2009, Volume 83, 1st edition dimdi.de (PDF), accessed on March 11, 2009
- ↑ JF Smith, M Brownlow, M Brown et al .: Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin 3: 109-115 (2007)
- ↑ a b H M Hepburn, AM Kaufmann: Nobel Prize for vaccination against cervical cancer. Current data and guidelines. In: Internist , 50, 2009, pp. 617-626, doi: 10.1007 / s00108-009-2388-9
- ↑ Rolando Herrero, Wim Quint, Allan Hildesheim, Paula Gonzalez, Linda Struijk, Hormuzd A. Katki, Carolina Porras, Mark Schiffman, Ana Cecilia Rodriguez, Diane Solomon, Silvia Jimenez, John T. Schiller, Douglas R. Lowy, Leen-Jan van Doorn, Sholom Wacholder, Aim e R. Kreimer, Torbj rn Ramqvist: Reduced Prevalence of Oral Human Papillomavirus (HPV) 4 Years after Bivalent HPV Vaccination in a Randomized Clinical Trial in Costa Rica. In: PLoS ONE. 8, 2013, p. E68329, doi: 10.1371 / journal.pone.0068329 .
- ↑ Doubts about the effectiveness . In: Frankfurter Rundschau , November 28, 2008
- ↑ Bielefeld Manifesto ( Memento from March 28, 2016 in the Internet Archive ) (PDF) by health scientists on HPV vaccination.
- ↑ a b Recommendation of the STIKO for HPV vaccination (PDF; 170 kB) In: Epidemiologisches Bulletin , 32/2009, 10 August 2009
- ↑ a b c European Medicines Agency: Cervarix product information texts (PDF) as of February 2014.
- ↑ Two-dose regimen for HPV vaccines. In: apotheke-adhoc.de. April 7, 2014, accessed June 10, 2018 .
- ↑ Yvonne Deleré et al .: Effectiveness and duration of vaccination protection against human papilloma viruses : Systematic literature review and meta-analysis. In: Dtsch Arztebl Int , 111, 2014, pp. 584–591, doi: 10.3238 / arztebl.2014.0584
- ↑ AR Kreimer et al .: Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. In: The Lancet, published online June 10, 2015 doi: 10.1016 / S1470-2045 (15) 70199-3
- ↑ Human Papillomavirus (HPV) ( Memento from February 23, 2016 in the Internet Archive ), Immunize Australia Program
- ^ Australian Government Department of Health: Government funds Gardasil.
- ↑ Public Health Agency of Canada: Human Papillomavirus (HPV) Prevention and HPV Vaccine: Questions and Answers
- ↑ Public Health Agency of Canada: Update On Human Papillomavirus (HPV) Vaccines
- ↑ Ministry of Health: Information on HPV vaccination in New Zealand ( Memento from January 30, 2009 in the Internet Archive )
- ^ Young-Tak Kim: Current status of cervical cancer and HPV infection in Korea. In: J Gynecol Oncol , 20, 2009, pp. 1-7, PMID 19471667 , doi: 10.3802 / jgo.2009.20.1.1
- ^ National Conference of State Legislatures: HPV Vaccine: State Legislation .
- ^ Jan Leidel: Statement to the state parliament of North Rhine-Westphalia. (PDF; 2.9 MB) from February 23, 2009
- ↑ Doris Uhl: Japan suspends recommendation for HPV vaccination. Deutsche Apothekerzeitung from June 20, 2013.
- ↑ Bulletin on drug safety. Federal Institute for Vaccines and Biomedical Medicines ( Paul Ehrlich Institute ), Issue 3, September 2013, No evidence of an increased risk of autoimmune diseases after HPV vaccination. Retrieved March 18, 2020 . (PDF; 1.1 MB).
- ↑ HPV vaccines: EMA confirms evidence does not support that they cause CRPS or POTS , November 5, 2015
- ↑ a b HPV Vaccines - EMA Confirmed: Evidence does not support the assumption that the vaccines cause CRPS or POTS . (PDF; 94 kB) January 12, 2016
- ↑ Satoshi Iwata, Kenji Okada, Kei Kawana: Consensus statement from 17 relevant Japanese academic societies on the promotion of the human papillomavirus vaccine. Vaccine 35 (2017), pp. 2291–2292, PMID 28325478 , doi: 10.1016 / j.vaccine.2017.03.015 , online (PDF document; 219 kB)
- ↑ Vaccination against human papillomavirus (HPV) for girls from 12 to 17 years - recommendation and justification . In: RKI (Ed.): Epidemiologisches Bulletin . No. 12 , March 23, 2007, p. 97-106 ( rki.de [PDF]).
- ↑ Scientists demand a reassessment of the HPV vaccination and an end to the misleading information. (PDF) November 25, 2008, archived from the original on April 1, 2014 ; Retrieved April 25, 2017 .
- ↑ Volker Stollorz: Vaccination and scolding . In: Die Zeit , No. 21/2009.
- ↑ Gerhardus, Ansgar et al .: Cervical cancer: How effective is the HPV vaccination? In: Dtsch Arztebl , 106, 2009, p. A-330 aerzteblatt.de
- ↑ Joachim Müller-Jung: Four professional associations defend cancer vaccination . In: FAZ , December 8, 2008; Retrieved June 30, 2009.
- ↑ Sonja Kastilian: An annoying manifesto . In: FAZ , December 8, 2008; Retrieved June 30, 2009.
- ↑ HPV vaccination: STIKO lowers age limit. In: univadis.de. August 26, 2014, archived from the original on June 12, 2018 ; accessed on June 12, 2018 .
- ↑ Epidemiological Bulletin 34/2014, STIKO recommendation on HPV vaccination (PDF) from August 25, 2014.
- ↑ Urologists recommend: The HPV vaccination for boys. In: urologenportal.de. DGU , BDU , June 23, 2016, accessed June 10, 2018 .
- ↑ Vaccination against human papillomavirus (HPV): Frequently asked questions and answers: Should boys also be vaccinated against HPV? Robert Koch Institute, June 7, 2018, accessed June 10, 2018 .
- ↑ Epidemiological Bulletin 26/2018, Scientific justification for recommending HPV vaccination for boys aged 9 to 14 years (PDF) from June 28, 2018.
- ^ Impfplan 2009 Austria - Evidence-based recommendations of the Supreme Sanitary Council ( Memento from November 5, 2013 in the Internet Archive ) (PDF; 452 kB), October 14, 2008.
- ↑ HPV vaccination: additional vaccination recommendation for boys and men aged 11 to 26 years . In: BAG , Federal Commission for Vaccination Issues (ed.): Bulletin . No. 10 , March 2, 2015, p. 140–149 ( admin.ch [PDF]).
- ↑ a b Human papillomavirus (HPV). Vaccination against cervical cancer. In: admin.ch. Federal Council , archived from the original on July 23, 2010 ; Retrieved July 23, 2010 .
- ↑ Sepp Leodolter: Mental and physical stress due to cancer precursors and conization: Combined prevention strategy could also prevent the majority of the cervical cancer precursors. Austrian initiative against cervical cancer, December 3, 2008
- ^ F Marra, K Cloutier, B Oteng, C Marra, G Ogilvie: Effectiveness and cost effectiveness of human papillomavirus vaccine: a systematic review. In: Pharmacoeconomics , 27, 2009, pp. 127-147, PMID 19254046 , doi: 10.2165 / 00019053-200927020-00004
- ↑ M. Jit et al .: Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ 337 (2008), 769, doi: 10.1136 / bmj.a769
- ^ Inge MCM de Kok, Marjolein van Ballegooijen, J. Dik F. Habbema: Cost-Effectiveness Analysis of Human Papillomavirus Vaccination in the Netherlands. Journal of the National Cancer Institute , doi: 10.1093 / jnci / djp183
- ^ Roxanne Nelson: HPV Vaccination Is Not Cost-Effective in the Netherlands. Medscape Medical News
- ↑ Jane J. Kim: Cost-Effectiveness of Human Papillomavirus Vaccination and Cervical Cancer Screening in Women Older Than 30 Years in the United States . In: Annals of Internal Medicine . 151, No. 8, October 19, 2009, pp. 538-545. Retrieved October 20, 2009.
- ↑ Prevention of the necessary cervical Ca precursors through vaccination is clearly proven. Interview with Harald zur Hausen. In: Ärzte Zeitung , December 3, 2008.
- ↑ Medical News Today, September 5, 2007 ( January 3, 2009 memento in the Internet Archive ), accessed February 22, 2016
- ^ The Economic Impact of AIDS in Kenya ( page 11 ) (PDF; 230 kB), The POLICY Project, 1999.
- ↑ Guideline of the Federal Joint Committee on Vaccinations according to Section 20d Paragraph 1 SGB V , last amended on December 5, 2013 (PDF; 244 kB).
- ↑ RKI - Recommendations of the STIKO - Advance information: STIKO recommends HPV vaccination for boys. Retrieved June 11, 2018 .
- ↑ Health insurers pay HPV vaccination for boys too , on spiegel.de
- ↑ Overview of health insurance companies that cover the costs of an HPV vaccination
- ↑ HPV vaccination free of charge , notification from the Federal Ministry of Health.
- ↑ Austrian initiative against cervical cancer .
- ↑ Ingrid Zechmeister, Birgitte Freiesleben de Blasio, Philipp Radlberger, Claudia Wild, Erich Kvas, Geoff Garnett, Aileen Rae Neilson. Economic evaluation of the vaccination against human papillomaviruses (HPV vaccination) in Austria. HTA project report 2007; 9. (PDF; 961 kB).
- ↑ Vaccination against human papillomavirus (HPV): costs are covered by compulsory health insurance . In: Federal Office of Public Health (Ed.): Bulletin . No. 8 , February 18, 2008 ( vaccination against human papillomavirus (HPV): costs are covered by the compulsory health insurance ( memento of April 26, 2009 in the Internet Archive ) [PDF]). Vaccination against human papillomavirus (HPV): costs are covered by the compulsory health insurance ( memento from 23 September 2015 in the Internet Archive )
- ↑ HPV vaccination programs in the cantons ( Memento from December 4, 2013 in the Internet Archive ) (PDF; 50 kB), P. Pesenti, media orientation from September 15, 2008.
- ↑ Reimbursement of costs for vaccinations against HPV , notification from the Federal Office of Public Health dated December 15, 2015.
- ↑ a b This page has been moved HPV - Vaccines - Vaccine Safety - CDC .
- ↑ Fédération Internationale de Gynécologie et d'Obstétrique : FIGO Statement on HPV Vaccination Safety. August 2, 2013, figo.org (PDF; 85 kB).
- ↑ Global Advisory Committees of the World Health Organization (WHO): Global Advisory Committee on Vaccine Safety Statement on the continued safety of HPV vaccination. March 12, 2014, who.int (PDF; 173 kB).
- ^ Francisco AR Garcia, Debbie Saslow: Prophylactic human papillomavirus vaccination: a breakthrough in primary cervical cancer prevention . In: Obstetrics and Gynecology Clinics of North America . tape 34 , no. 4 , December 2007, pp. 761-781, ix , doi : 10.1016 / j.ogc.2007.09.007 , PMID 18061868 .
- ↑ Nikolai Madrid Scheller, Björn Pasternak, Henrik Svanström, Anders Hviid: Quadrivalent human papillomavirus vaccine and the risk of venous thromboembolism . In: JAMA . tape 312 , no. 2 , July 2014, p. 187-188 , doi : 10.1001 / jama.2014.2198 , PMID 25005658 .
- ↑ Nikolai Madrid Scheller, Henrik Svanström, Björn Pasternak, Lisen Arnheim-Dahlström, Karin Sundström, Katharina Fink, Anders Hviid: Quadrivalent HPV Vaccination and Risk of Multiple Sclerosis and Other Demyelinating Diseases of the Central Nervous System. JAMA 313 (2015), pp. 54-61, doi: 10.1001 / jama.2014.16946 .
- ↑ Serena Colafrancesco, Carlo Perricone, Lucija Tomljenovic, Yehuda Shoenfeld: Human papilloma virus vaccine and primary ovarian failure: another facet of the autoimmune / inflammatory syndrome induced by adjuvants. (PDF) American Journal of Reproductive Immunology 70 (2013), 309-16, PMID 23902317 , doi: 10.1111 / aji.12151
- ↑ Louise Brinth: Responsum to Assessment Report on HPV vaccines released by EMA November 26th 2015. , online (PDF; 1.3 MB)
- ^ Nordic Cochrane Center: Complaint to the European Medicines Agency (EMA) over maladministration at the EMA (PDF) , from May 26, 2016.
- ↑ Arbyn M, Xu L, Simoens C, Martin-Hirsch PPL: Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No .: CD009069. DOI: 10.1002 / 14651858.CD009069.pub3 .
- ↑ Jørgensen L, Gøtzsche PC, Jefferson T .: The Cochrane HPV vaccine review was incomplete and ignored important evidence of bias. BMJ Evidence-Based Medicine 2018; 23, pp. 165-168, DOI: 10.1136 / bmjebm-2018-111012
- ^ Statement from Cochrane's Governing Board
- ↑ Statement from Cochrane's Governing Board - September 26th, 2018
- ↑ HPV vaccination during pregnancy without negative consequences. Deutsches Ärzteblatt , March 31, 2017, accessed on September 9, 2019 .
- ↑ Ulrich Heininger: Impfratgeber. Vaccination recommendations for children, adolescents and adults. 5th edition. Uni-Med Verlag, Bremen 2009, ISBN 978-3-8374-1101-0 . Pp. 99-102.
- ↑ Epidemiological Bulletin 12/2007, recommendation of the STIKO on HPV vaccination of March 23, 2007 (PDF; 120 kB).
- ↑ HPV Vaccination - Answers to Common Questions. DKFZ , July 22, 2019, accessed on September 9, 2019 .
- ↑ CM Corona Gutierrez et al .: Therapeutic Vaccination with MVA E2 Can Eliminate Precancerous Lesions (CIN 1, CIN 2, and CIN 3) Associated with Infection by Oncogenic Human Papillomavirus . In: Human Gene Therapy . tape 15 , no. 5 , 2018, p. 421-431 , doi : 10.1089 / 10430340460745757 , PMID 15144573 .
- ↑ Clinical study (phase III): REVEAL 1 (Evaluation of VGX-3100 and Electroporation for the Treatment of Cervical HSIL) (REVEAL 1) at Clinicaltrials.gov of the NIH .
- ↑ Vaccination as therapy: experimental vaccine against cervical cancer successfully tested in mice. Press release from the German Cancer Research Center from January 16, 2019
- ↑ Sebastian Kruse et al .: Therapeutic vaccination using minimal HPV16 epitopes in a novel MHC-humanized murine HPV tumor model . In: OncoImmunology . tape 8 , no. 1 , January 2, 2019, p. e1524694 , doi : 10.1080 / 2162402X.2018.1524694 , PMID 30546964 , PMC 6287800 (free full text).