T cell receptor
The T cell receptor (engl. T cell receptor , TCR ) is a protein complex , which on the surface of T-cells is anchored, and for the detection of antigens , by MHC , is responsible molecules are presented. It has the special property of distinguishing foreign from endogenous antigens and only activates the T cell in the first case. The binding of a matching antigen foreign to the body causes a change in gene expression via the TCR signal path , which leads to cell proliferation , secretion of cytokines , surface expression of costimulators and differentiation into effector and memory cells .
structure
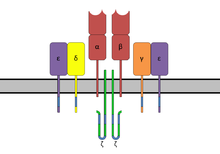
The TCR is structurally a Fab fragment of the antibody very similar as its subunits also from the superfamily immunoglobulin derived. The TCR complex consists of two protein subunits (mostly α / β, γ / δ in about 5% of cells), which in turn each consist of a constant domain (C) and a variable domain (V), a transmembrane domain and a short one C-terminal cytoplasmic area exist. The N-terminal ends of the chains, which belong to the C domain, penetrate the cell membrane into the cytoplasmic space and anchor the receptor. The two subunits are connected to each other extracellularly via a disulfide bridge in the constant region.
The variable domains of the α and β subunits are composed of V and J segments or, for the β chain, of V, D and J segments, each of which is three hypervariable (and on the β chain with HV4 form a fourth, but without antigen contact) and thus binding-critical regions (English complementarity determining regions , CDRs). The CDR 2 interacts primarily with the α-helices on the peptide bond cleavage of the MHC class I and II complexes, while the CDR 1 of the α chain at the N-terminus and the CDR 1 of the β chain at the C- Terminus of the antigen binds and the two CDR3 segments contribute the largest share to the binding of the MHC-presented antigen. The latter show a greater diversity mainly due to the combinatorial diversity in the V (D) J recombination . The CDR4 does not interact with antigens, but interactions with superantigens have been described.
Whether or not a TCR can bind an antigen is a very complex process. In general, the lock and key principle applies , so if the structure of a presented antigen in connection with the presenting MHC molecule matches the α and β chains of the TCR, binding occurs. Computer-aided molecule and bond simulations are one of the tasks of bioinformatics . The specificity of the TCR can be determined by epitope mapping .
Emergence
In addition to the structure, the development of T-cell receptors is similar to that of B-cell antibodies . The two subunits arise through genetic V (D) J recombination (VJ in the α or γ chain, VDJ in the β or δ chain). The genes are arranged almost randomly in order to guarantee the greatest possible diversity. In addition, insertions of N or P nucleotides take place . This is the basic building block of the adaptive immune response in T cells. The T-cell clones, each with a unique T-cell receptor, are subject to positive and negative selection in the thymus in order to sort out T-cells with non-functional or self-reactive products of the recombination.
Anti-gene discrimination
A unique property of the T cell receptor is the ability to distinguish foreign antigens from pathogens and cancer cells from the body's own antigens. Antigen-presenting cells do not differentiate between cells foreign to the body and cells produced by the body. In addition to a few foreign antigens, they present a large number of endogenous antigens on their cell surface. The T-cell receptor is able to be activated by the few foreign antigens to which it binds with high affinity , but not by the multitude of endogenous antigens to which it binds only with low affinity. This phenomenon is known as antigen discrimination . The T cell receptor is both very specific and very sensitive to enable this antigen discrimination. The activation of the T cells correlates with the affinity of the T cell receptor for the antigen-MHC complex. The TCR is so specific that it can even recognize the change in a single amino acid in the antigen. This loss in affinity cannot be compensated for even by higher antigen concentrations. Despite the great specificity, the receptor has a very high sensitivity. The interaction with a single antigen-MHC complex is sufficient to activate the T cell.
The mechanism of these actually mutually exclusive properties of specificity and sensitivity is not yet fully understood. Stoichiometric studies on TCRs and the associated CD3 subunits on the surfaces of living T cells show that monomeric TCR-CD3 complexes are the basis for the recognition of the antigenic pMHCs.
Signal transduction
Since the T cell receptor itself only has a very short cytoplasmic area, it forms a complex with CD3 adapter proteins, which have signal transduction motifs, so-called immunoglobulin tyrosine activation motifs (ITAMs), in their long cytoplasmic amino acid chain. In mammals, CD3 consists of γ, δ, and ε chains as well as complexes of ζ 2 or - / η chains. The entire complex is referred to as the T cell receptor complex . After binding of an antigen-MHC complex, activation of the TCR and binding of the co-receptor CD4 or CD8 to MHC, tyrosine kinases Lck and Fyn phosphorylate tyrosine residues in the ITAMs. The tyrosine phosphatase CD45 can remove the phosphoylations again and thus switch off the TCR signal. At the same time, CD45 can also activate Lck and Fyn through dephosphorylation. The phosphorylated ITAMs serve as binding sites for ZAP-70 , which is brought into the vicinity of Lck through this binding. Lck phosphorylates ZAP-70, which can now phosphorylate the adapter protein LAT in several places. LAT is a scaffold protein , it has catalytic activity itself, but serves as a binding partner for many other signal proteins. This creates a large protein complex that can activate the following signaling pathways. Among other things, various MAP kinase pathways are activated that lead to the activation of the transcription factor AP-1 . Furthermore, the activation of the phospholipase C -γ (PLC-γ) can take place by binding to LAT. PLC-γ produces the second messenger diacylglycerol (DAG) and inositol trisphosphate (IP 3 ), which activate the transcription factors NF-κB or NFAT via calcium influx into the cell.
Co-receptors
The signal resulting from the TCR antigen binding is amplified by the simultaneous binding to co-receptors. The CD4 and CD8 receptors bind to the MHC molecule after the TCR binds to the antigen. Lck, which binds to the cytoplasmic domain of the co-receptors, is brought close to the TCR. The CD4 receptor binds exclusively MHC II , while the CD8 specific for MHC I is. CD4 and CD8 determine the differentiation into T helper cells and cytotoxic T cells during development in the thymus. The binding of the co-receptors also has an effect on the specificity and sensitivity of the T cell receptor.
Binding of co-stimulatory receptors and their ligands on antigen-presenting cells increases the activation of the T cell. One example is the CD28 receptor on T cells, which binds the proteins of the B7 family ( CD80 and CD86 ). Antigen-presenting cells express the B7 proteins on their surface when, as part of the innate immune system, they have recognized an infection with a pathogen. The co-stimulating receptors deliver a second signal in addition to the TCR, so to speak a signal that signals danger, and only if this is present in addition to the recognition of an antigen by the TCR can the T cell be activated. This system ensures that T cells that accidentally recognize an endogenous antigen are not activated. However, some co-stimulating receptors are not absolutely necessary, but only strengthen the TCR signal.
There are also co-inhibitory receptors, e.g. B. CTLA-4 and PD-1 , which decrease or block the TCR signal. They play an important role in regulating and switching off the immune reaction once the pathogen has been successfully fought.
literature
- Janeway et al .: Immunobiology . 6th edition ISBN 0815341016 . The 5th English edition is available online on the pages of the NCBI Bookshelf (online) .
Web links
- Jennifer McDowall / Interpro: Protein Of The Month: T-cell receptors. (engl.)
Individual evidence
- ↑ a b Feinerman O, Germain RN, Altan-Bonnet G: Quantitative challenges in understanding ligand discrimination by alphabeta T cells . In: Molecular Immunology . 45, No. 3, February 2008, pp. 619-31. doi : 10.1016 / j.molimm.2007.03.028 . PMID 17825415 . PMC 2131735 (free full text).
- ↑ Blum JS, Wearsch PA, Cresswell P: Pathways of antigen processing . In: Annual Review of Immunology . 31, 2013, pp. 443-73. doi : 10.1146 / annurev-immunol-032712-095910 . PMID 23298205 . PMC 4026165 (free full text).
- ↑ Dushek O, Aleksic M, Wheeler RJ, Zhang H, Cordoba SP, Peng YC, Chen JL, Cerundolo V, Dong T, Coombs D, van der Merwe PA: Antigen potency and maximal efficacy reveal a mechanism of efficient T cell activation . In: Science Signaling . 4, No. 176, June 2011, p. Ra39. doi : 10.1126 / scisignal.2001430 . PMID 21653229 . PMC 4143974 (free full text).
- ↑ Altan-Bonnet G, Germain RN: Modeling T cell antigen discrimination based on feedback control of digital ERK responses . In: PLoS Biology . 3, No. 11, November 2005, p. E356. doi : 10.1371 / journal.pbio.0030356 . PMID 16231973 . PMC 1262625 (free full text).
- ↑ Huang J, Brameshuber M, Zeng X, Xie J, Li QJ, Chien YH, Valitutti S, Davis MM: A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4 (+) T cells . In: Immunity . 39, No. 5, November 2013, pp. 846-57. doi : 10.1016 / j.immuni.2013.08.036 . PMID 24120362 . PMC 3846396 (free full text).
- ↑ Mario Brameshuber, Florian Kellner, Benedikt K. Rossboth, Haisen Ta, Alge Kevin, Eva Sevcsik, Janett Göhring, Markus Axmann, Florian Baumgart, Nicholas RJ Gascoigne, Simon J. Davis, Hannes Stockinger, Gerhard J. Schütz, Johannes B. Huppa: Monomeric TCRs drive T cell antigen recognition . In: Nat. Immunol . April 16, 2018. doi : 10.1038 / s41590-018-0092-4 .
- ↑ Call ME, Pyrdol J, Wiedmann M, Wucherpfennig KW: The organizing principle in the formation of the T cell receptor-CD3 complex . In: Cell . 111, No. 7, December 2002, pp. 967-79. doi : 10.1016 / s0092-8674 (02) 01194-7 . PMID 12507424 . PMC 3420808 (free full text).
- ↑ Clare L. Abram, Clifford A. Lowell: The Expanding Role for ITAM-Based Signaling Pathways in Immune Cells . In: Science Signaling . 2007, No. 377, March 13, 2007, p. Re2.
- ↑ a b Janeway's immunobiology , Ninthition. Edition, ISBN 0815345518 .
- ↑ JD Stone, AS Chervin, DM Kranz: T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. . In: Immunology . 126, No. 2, February 2009, pp. 165-76. doi : 10.1111 / j.1365-2567.2008.03015.x . PMID 19125887 .
- ^ A b c Lieping Chen, Dallas B. Flies: Molecular mechanisms of T cell co-stimulation and co-inhibition . In: Nature Reviews Immunology . 13, No. 4, March 8, 2013, pp. 227–242. doi : 10.1038 / nri3405 .