Superantigen

Superantigens (SAG) are antigens that lead to an antigen-independent activation of T lymphocytes and thus act as toxins .
properties

Superantigens are the most potent activators of T lymphocytes and are associated with a variety of diseases that can be attributed to T cell toxicity . In normal antigens, the formation of cell-cell contacts between MHC- bound peptides on antigen-presenting cells and T-cell receptors (TCR) on T-lymphocytes leads to the initiation of an adaptive immune response . In the case of superantigens, on the other hand, this T lymphocyte activation takes place antigen-unspecifically and bypassing the processing by antigen-presenting cells, in that the superantigen acts as a bridge connecting receptors : While conventional antigens react with only 0.01% to 1% of all T cells, this proportion is included Superantigens at 5% to 20% (see below). The subsequently polyclonally increased T cells emit cytokines in an almost uncontrolled manner , which thwarts a targeted immune defense. For the superantigen SMEZ-2 produced by streptococci , an activity in the femtogram range per milliliter can be demonstrated, making it the most potent of the bacterial superantigens to date (2005).
Structure of the superantigens
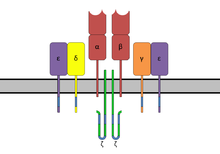
SAG are bifunctional molecules that can simultaneously connect the receptor binding sites of class II MHC molecules of the antigen presenting cells ( APC ) with the T cell receptor Vβ elements ( TCR Vβ) and CD28 of the T cells. This allows them to negatively modulate the interaction between class II MHC molecules and TCR by forming a trimer (MHC-II / SAG / TCR). Last but not least, the structure of the superantigens is the reason for their high stability against proteases and heat. In contrast to conventional antigens , the epitopes of which have a size between about eight and seventeen amino acids , bacterial SAGs are proteins with a size of 20-30 kDa in terms of their molar mass . In streptococci, superantigens are usually found in a size of 24–28 kDa. Most SAGs are globular proteins . Structural analysis showed that they consist of a single chain formed from two globular domains . It is assumed that the superantigens of the staphylococci and streptococci have common origins, especially since one can find matches of 20% to 90% when comparing amino acid sequences .
Superantigens than conventional antigens
Superantigens also act to a lesser extent as antigens in the context of an adaptive immune response. The immunoglobulins IgE and IgG against SAGs can also be detected in patient sera. Using the example of the staphylococcal enterotoxins A, B and TSST-1, it was possible to show that an IgE-mediated release of histamine takes place in this way and thus inflammatory reactions are intensified. In other experiments, the as yet unexplained effect occurred that a constitutive expression of SPEA (Streptococcal Pyrogenic Exotoxin A) took place in patients with an S. pyogenes infection . Due to the permanent formation of SPEA, it is theoretically possible for the affected organism in the long term to form neutralizing antibodies against the toxin. However, in the case of SPEA, no antibody formation could be detected in the course of an acute illness, which can result in a more dramatic course. The fact that superantigens can also act as classic antigens is interesting in that a vaccination can be carried out with small amounts or toxoids . This approach could be verified on the animal model. The prognosis for the course of the disease could therefore be more positive.
Types of binding to target cells
To interact with the target cells, SAGs have at least two class II MHC molecule binding sites that differ among the SAGs. A low- affinity binding site on the α-chain and / or high-affinity, zinc-dependent binding site on the β-chain of class II MHC molecules are available for binding. The zinc-dependent binding site can be decisive for the activity of the SAG. SAG that are capable of a zinc bond have the primary zinc bond motif HXD at the C-terminal , consisting of the amino acids histidine (H), any one (X) and aspartic acid (D). In the case of the superantigens SPEC, SPEGGAS, SPEH, SPEJ and SMEZ, for example, the zinc bond on the polymorphic β chain of the class II MHC molecules is dominant. The only known streptococcal SAGs without zinc binding are SSA and SPEA. The staphylococcal enterotoxins A (SEA) and E (SEE) are known to combine both forms of attachment.
Interaction with target cells
The interaction with the T cells takes place mainly via the CDR2 and HV4 regions of the Vβ elements of the TCR , partly with a slight influence of other variable regions of the TCR. This distinguishes SAG in its binding compared to conventional antigens, which use the complementarity determining region (CDR) for this. The Vβ elements are limited to around 50 genes in humans . Of these, a strong interaction with SAG is currently (2005) described for only about 24 types. All SAGs have a specific profile of different recognition sequences of Vβ elements and thus bind to those T cells which express the corresponding Vβ elements, regardless of the antigen specificity. This enables SAG to interact with 5% to 20% of all T cells, resulting in hyperproliferation . In contrast, conventional antigens react with 0.01% to 1% of all T cells.
In conventional antigens takes place after a first recording of their exogenous processing into small peptide fragments 9-30 amino acids within the lysosomal compartment of the phagosome of the antigen-presenting cell. After fusion of the phagosome and endosome containing Class II MHC molecules to present antigen fragments takes place at the cell surface. As a result, a specific and controlled immune reaction with proliferation of T cells, the release of cytokines and antibody formation is induced. Superantigens, on the other hand, are independent of processing and presentation and do not bind in the binding pit, but directly outside to the DR or DQ domain of the class II MHC molecules of the APC (B cells, dendritic cells, monocytes ). There is no MHC restriction , as is typically found with peptide antigens.
Consequences of activation
The superantigen-mediated activation of the APC and T cells leads to massive systemic lymphokine discharges within the first few hours . This leads to an increase in the blood values of the cytokines IL-1 , IL-2 , IL-6 , TNF-α , gamma interferon (IFN-γ), macrophage inflammatory protein 1α (MIP-1α), MIP-1β and monocyte chemoattractant protein 1 ( MCP-1 ). The interaction of SAG and class II MHC molecules leads to the activation of phospholipase C and protein kinase C, increased cytokine expression (IL-1, IL-12, TNF-α) and phosphoinositol breakdown. The activation of T cells leads to a class change in B cells via the formation of CD40 . The abnormal release of lymphokines appears to be the cause of Toxic Shock Syndrome (TSS) and a number of other diseases.
Bacterial superantigens
Most superantigens are made by bacteria , especially gram-positive bacteria. The superantigens TSST (Toxic Shock Syndrome Toxin) from Staphylococcus aureus and SPE (Streptococcus pyogenes exotoxin) from invasive Streptococcus pyogenes are widely considered to be highly lethal toxins for humans . About 1% of Staphylococcus aureus form TSST-1, which leads to septic shock via interleukin-1 and TNF-α release from macrophages. Other strains produce a so-called exfoliative toxin as SAG and thus cause Ritter's disease ( staphylococcal scalded skin syndrome , SSSS) in small children . Groups C and G streptococci have also been identified as potential toxin generators, but their importance seems to be limited to animals. Among the gram-negative microorganisms, the toxins MAM and YPM from Mycoplasma arthritidis and Yersinia pseudotuberculosis have been identified as superantigens.
Viral superantigens
In addition to the bacterial superantigens, superantigenic effects in infections with viruses of the herpes family , the mouse mammary tumor virus (MMTV), and the human immunodeficiency virus 1 ( HIV- 1) are suspected.
Importance of superantigens for the microorganism
While pathogens often avoid an immune reaction in the course of an immune evasion , the production of superantigens and the resulting excessive permanent activation of immune cells also serve to hinder the adaptive immune reaction. Presumably, the superantigen-mediated stimulation leads to the consumption of locally produced interleukin-2 by T cells, which suppresses an effective immune response against pathogens. The massive cytokine release presumably also leads to a delayed specific recognition of the pathogen, which facilitates its spread. Furthermore, SAG can cause anergy and / or deletion of T cells, which also leads to an effective protection of the pathogen from the adaptive immune response. There are in-vitro studies of the streptococcal pyrogens exotoxin A and C and the staphylococcal enterotoxin B which show that these toxins can suppress the humoral immune response in certain cases and thus also prevent the formation of antibodies. The transcription regulation of the genes of the superantigens is often carried out by the same factors as the transcription of the M proteins and proteins of the bacterial capsule.
The involvement of superantigens in diseases
Diseases suspected of being associated with superantigens
Various clues point to the involvement of SAG in certain diseases or are even considered as their etiology . However, the role of SAG has not yet been proven for many diseases. In insulin-dependent diabetes mellitus a relationship to T-cell superantigen IDDMK is 1.2 of the human-Endogenous retrovirus (HERV) 22 K-18 and enterotoxins from S. aureus discussed. The same applies to autoimmune reactions such as rheumatic endocarditis , Sjogren's syndrome , acute rheumatic fever , rheumatoid arthritis , Kawasaki syndrome and multiple sclerosis , which are suspected to be related to bacterial SAG. The pyrogenic exotoxins A, C, G through J, SSA and variants of SMEZ appear to play a significant role in acute tonsillitis , necrotizing fasciitis , rheumatic fever, and scarlet fever . SAGs have the same unexplained role in sudden infant death syndrome (SIDS), in which after autopsies it is conspicuously often possible to isolate S. aureus strains that produce pyrogenic exotoxins. It is assumed that SAG also have an influence on some skin diseases such as atopic eczema , atopic dermatitis and psoriasis , since significantly increased amounts of SAG could be isolated from patient samples. For SPE A and C it could be shown that these are related to psoriasis guttata.
Diseases related to superantigens
On the other hand, is undisputed that the enterotoxins A to M and TSST-1 by S. aureus , the toxic shock syndrome (TSS) followed by multiple organ failure as well as various food poisoning (TSST-1 except for) can cause. The same applies to streptococcal SAG, which are also called pyrogenic exotoxins and u. a. cause streptococcal-induced toxic shock syndrome and scarlet fever (see above).
literature
- Charles Janeway et al .: Immunobiology . 6th edition. ISBN 0815341016 . The 5th English edition is available online on the pages of the NCBI Bookshelf (online) .
Individual evidence
- ↑ G. Ramachandran: Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. In: Virulence. Volume 5, Number 1, January 2014, pp. 213-218, ISSN 2150-5608 . doi : 10.4161 / viru.27024 . PMID 24193365 . PMC 3916377 (free full text).
- ↑ C. Louis-Dit-Sully, B. Blumenthal, M. Duchniewicz, K. Beck-Garcia, GJ Fiala, E. Beck-García, M. Mukenhirn, S. Minguet, WW Schamel: Activation of the TCR complex by peptide -MHC and superantigens. In: EXS. Volume 104, 2014, pp. 9-23, ISSN 1023-294X . doi : 10.1007 / 978-3-0348-0726-5_2 . PMID 24214615 .
- ^ R. Kaempfer, G. Arad, R. Levy, D. Hillman, I. Nasie, Z. Rotfogel: CD28: direct and critical receptor for superantigen toxins. In: Toxins. Volume 5, Number 9, September 2013, pp. 1531-1542, ISSN 2072-6651 . doi : 10.3390 / toxins5091531 . PMID 24022021 . PMC 3798871 (free full text).
- ^ IV Pinchuk, EJ Beswick, VE Reyes: Staphylococcal enterotoxins. In: Toxins. Volume 2, Number 8, August 2010, pp. 2177-2197, ISSN 2072-6651 . doi : 10.3390 / toxins2082177 . PMID 22069679 . PMC 3153290 (free full text).
- ↑ a b Stiles BG, Krakauer: staphylococcal enterotoxin: a purging Experience in Review, Part I . In: Clinical Microbiology Newsletter . 27, 2005, p. 23.
- ↑ a b Jabara HH, Geha RS: The superantigen toxic shock syndrome toxin-1 induces CD40 ligand expression and modulates IgE isotype switching . In: Int. Immunol. . 8, No. 10, October 1996, pp. 1503-10. doi : 10.1093 / intimm / 8.10.1503 . PMID 8921429 .
- ^ AR Spaulding, W. Salgado-Pabón, PL Kohler, AR Horswill, DY Leung, PM Schlievert: Staphylococcal and streptococcal superantigen exotoxins. In: Clinical microbiology reviews. Volume 26, Number 3, July 2013, pp. 422-447, ISSN 1098-6618 . doi : 10.1128 / CMR.00104-12 . PMID 23824366 . PMC 3719495 (free full text).
- ↑ Thomas Proft et al .: Superantigens and Streptococcal Toxic Shock Syndrome . Emerging Infectious Diseases 9, Oct. 2003, doi : 10.3201 / eid0910.030042 .
- ↑ Klaus-Peter W. Schaps u. a. Ed .: The second - compact: Basics . Springer, 2008, ISBN 978-3540463443 .
- ↑ Jean-François Fortin et al .: Hyper-responsiveness to stimulation of human immunodeficiency virus-infected CD4 + T cells requires Nef and Tat virus gene products and results from higher NFAT, NF-kappaB, and AP-1 induction . J Biol Chem. 278, Sept. 2004, pp. 39520-31, doi : 10.1074 / jbc.M407477200 .
- ↑ Satinder Dahiya et al .: Deployment of the human immunodeficiency virus type 1 protein arsenal: combating the host to enhance viral transcription and providing targets for therapeutic development , J Gen Virol 93, June 2012, pp. 1151–1172, doi : 10.1099 / vir.0.041186-0 .
- ↑ Lussow AR, MacDonald HR: Differential effects of superantigen-induced "anergy" on priming and effector stages of a T cell-dependent antibody response . In: Eur. J. Immunol. . 24, No. 2, February 1994, pp. 445-9. doi : 10.1002 / eji.1830240227 . PMID 8299694 .
- ↑ Cleary PP, McLandsborough L, Ikeda L, Cue D, Krawczak J, Lam H: High-frequency intracellular infection and erythrogenic toxin A expression undergo phase variation in M1 group A streptococci . In: Mol. Microbiol. . 28, No. 1, April 1998, pp. 157-67. doi : 10.1046 / j.1365-2958.1998.00786.x . PMID 9593304 .