Epitope
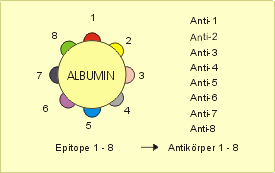
An epitope (Greek ἐπί epi "at, on" and τόπος topos "place", synonymous: antigenic determinant ) is a structure against which antibodies or T-cell receptors are formed in the course of an adaptive immune response . More precisely, epitopes are circumscribed molecular structures or molecular segments of an antigen that can trigger a specific immune response.
Often it is an area of the surface of an antigen to which an antibody or T cell receptor specifically binds. Antibodies are normally directed against proteins or proteids . In rare cases, antibodies against other chemical structures (e.g. DNA , saccharides , heavy metals , steroid hormones , penicillin ) can also be formed.
properties
A single antigen, such as B. a membrane protein of a cell or a bacterium , carries different spatial epitopes. A specific antibody or a specific T cell can be formed against each of these epitopes . Epitopes can be species-specific or individual-specific. After the antigens have been phagocytosed by antigen-presenting cells , these present the epitopes on their cell surface. Then the adaptive immune response takes place .
Typically, epitopes which are bound to MHC class I molecules have a length of 8 to 11 amino acids, whereas MHC class II epitopes have longer peptide chains of 9 to 18 amino acids.
A distinction is made between continuous (synonymous sequence epitopes) and discontinuous epitopes (synonymous conformational epitopes). Proteins consist of amino acid chains and are folded in three dimensions . Epitopes can consist of different amino acid residues that are close together in space, but which are actually far apart in terms of the amino acid sequence. Such epitopes are called discontinuous because they are only present and can be recognized in the native state of the protein. In SDS gel electrophoresis and a subsequent Western blot, for example, the amino acid chains are unfolded ( denaturation ) and the antibodies no longer bind to discontinuous epitopes if the epitopes could not fold again into the correct, native shape. Continuous epitopes, on the other hand, remain even after denaturation, since they consist of a sequence of consecutive amino acids, which is retained during denaturation.
The binding specificity of an antibody to its epitope is referred to as affinity , while the sum of the binding energies of all antibodies binding to an antigen (with several epitopes) is referred to as avidity . The area on the bound antibody or T-cell receptor opposite the epitope at the binding site is called the paratope . The immunodominant epitopes generate a stronger immune response.
application
In genetic engineering , epitope tags are used (e.g. His-Tag, Myc-tag, Strep-Tag, V5-Tag or Flash-tag) in order to enable the detection of proteins as fusion proteins for which no suitable antibody is available stands. For this purpose, the gene of an epitope is incorporated into the same reading frame as the protein to be examined , so that the expressed protein receives an N- or C-terminal epitope tag . An antibody can now be used for detection against the attached epitope . The same epitope can be added to new proteins again and again in different experiments, and thus the same antibody can always be used, which represents a considerable saving in terms of effort and costs.
The Immune Epitope Database is an online database for epitopes that have already been described, and programs for predicting the binding affinity of epitopes to MHC molecules are also offered ( reverse immunology ). The systematic study of the epitopes in an antigen is called epitope mapping .
literature
- Janeway et al .: Immunobiology . 6th edition ISBN 0-8153-4101-6 . The 5th English edition is available online on the pages of the NCBI Bookshelf (online) .