carbohydrates
Carbohydrates or saccharides form a biochemically important class of substances . Carbohydrates are found in the metabolism of all living things. As a product of photosynthesis , carbohydrates make up around two thirds of the world's biomass . Carbohydrates are the most common class of biomolecules . The science that deals with the biology of carbohydrates and carbohydrate metabolism is called glycobiology . Carbohydrates are often identified with the suffix " -ose ".
etymology
Carbohydrates are not hydrates
Since many saccharides the empirical formula C n (H 2 O) m have been incorrectly assumed that this was hydrates handle of the carbon, which is why Carl Schmidt 1844 the term carbohydrates coined which until today as coal n hydrate is used in a modified form. Representatives of this substance class can, however, deviate considerably from this gross formula and contain further functional groups and heteroatoms such as nitrogen or sulfur, while other compounds of the same formula do not belong to the carbohydrates, since they are not hydroxyaldehydes or hydroxyketones. In general, carbohydrates are present when at least one aldehyde group or keto group and at least two hydroxyl groups can be found in a substance . The following formula applies to unbranched polysaccharides that are built up from the same monosaccharide with the sum formula C 6 H 12 O 6 ( glucose , fructose , galactose, etc.):
The name saccharide comes from the Greek word σάκχαρον ( sákkharon ) for sugar.
history

As early as 1811, Constantin Kirchhoff made the discovery that when starch is cooked with acid, glucose is formed. At the suggestion of Johann Wolfgang Döbereiner , a starch sugar factory was built in 1812 during the continental blockade . Henri Braconnot discovered in 1819 that the action of concentrated sulfuric acid on cellulose produces sugar. William Prout gave this group of substances the group name Sacharine after chemical analyzes of sugar and starch by Joseph Louis Gay-Lussac and Thénard . Chemists who have been involved in the research of carbohydrates include Emil Fischer (1852-1919), Burckhardt Helferich (1887-1982), Bernhard Tollens (1841-1918), Walter Norman Haworth (1883-1950) and Wilhelm Koenigs ( 1851–1906) with his colleague Eduard Knorr (1867–1926) ( Koenigs-Knorr method ).
Emil Fischer received the Nobel Prize in Chemistry in 1902 for his work on sugars and purines . Otto Meyerhof received the Nobel Prize for Physiology or Medicine in 1922 for his discovery of the metabolism of glucose . Together with Arthur Harden, Hans von Euler-Chelpin received the Nobel Prize in Chemistry in 1929 “for their research on sugar fermentation and the role of enzymes in this process”. In 1947, Bernardo Houssay received the Nobel Prize in Physiology or Medicine for his discovery of the role of the pituitary gland in the metabolism of carbohydrates, and Carl and Gerty Cori for their discovery of the conversion of glycogen . Luis Leloir received the Nobel Prize in Chemistry in 1970 for the discovery of sugar nucleotides in the biosynthesis of carbohydrates .
properties
A carbohydrate has the general empirical formula C n H 2n O n with n ≥ 3. Alternatively, carbohydrates can be defined as polyhydroxyaldehydes and ketones and molecules that result in such after hydrolysis - but their derivatives such as deoxyribose are also referred to as carbohydrates, which are different Have a molecular formula. The IUPAC defines carbohydrates including sugar alcohols , sugar acids , deoxy sugars , amino sugars , thio sugars and similar compounds.
Systematics
Carbohydrates come in different chain lengths (also as polymers ) and are therefore divided into mono-, di-, tri-, oligo- and polysaccharides. The monosaccharides ( simple sugars such. As glucose , fructose ), disaccharides ( double sugars , for. Example, granulated sugar , lactose , maltose ) and oligosaccharides ( complex sugars , with <10 monosaccharide units, eg. As stachyose , raffinose ) are water soluble, have have a sweet taste and are called sugar in the narrower sense . The polysaccharides ( multiple sugars such. As starch ( amylose and amylopectin ), cellulose, chitin and animals glycogen ) are often poor contrast or not soluble in water and tasteless. Monosaccharides with three carbon atoms are called triose, with four carbon atoms as tetrose, with five carbon atoms as pentose, with six carbon atoms as hexose etc. Polymers of carbohydrates with only one basic building block (more precisely: with only one type Monosaccharide) are referred to as homoglycans , while polymeric carbohydrates from various basic components are referred to as heteroglycans .
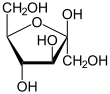
There are two possible structures in the trioses, since a carbohydrate always has an aldehyde or a keto group, each in a D and an L form: glyceraldehyde (as aldotriose) and dihydroxyacetone (as triulose). The tetroses have three representatives: as aldotetroses, erythrose and threose , and as tetrulose, erythrulose . Among the pentoses there are ribose , arabinose , xylose and lyxose as aldopentoses and ribulose and xylulose as pentuloses . The aldohexoses (i.e. carbohydrates with 6 carbon atoms and one aldehyde group) are divided into eight possible representatives. These are sorted according to the Fischer nomenclature: Allose , Altrose , Glucose, Mannose , Gulose , Idose , Galactose and Talose . The four possible hexuloses (i.e. carbohydrates with 6 carbon atoms and one keto group) are sorted according to the Fischer nomenclature: psicose , fructose, sorbose and tagatose .
Occurrence
Carbohydrates are found in all living things and are a central component of energy metabolism . Monosaccharides are naturally found in higher concentrations in fruits and honey . Among the disaccharides, sucrose is found in sugar beet and sugar cane . The disaccharide lactose is found in milk and dairy products, while maltose occurs naturally in honey and artificially in malt and starch sugar . Raffinose and stachyose are found in relatively higher concentrations in cereals , tubers , onions and malt. Starch and dextrins are accumulated from grain, roots, tubers and vegetables . Celluloses, hemicelluloses, are produced by all plants. Cellulose is the most common biomolecule. Accordingly, glucose, from which cellulose is made, is the most common monosaccharide.
| food | Total carbohydrates including fiber |
Total sugar | Fructose | glucose | Sucrose | Fructose / glucose ratio |
Sucrose in% of total sugar |
|---|---|---|---|---|---|---|---|
| fruit | |||||||
| Apple | 13.8 | 10.4 | 5.9 | 2.4 | 2.1 | 2.0 | 19.9 |
| apricot | 11.1 | 9.2 | 0.9 | 2.4 | 5.9 | 0.7 | 63.5 |
| banana | 22.8 | 12.2 | 4.9 | 5.0 | 2.4 | 1.0 | 20.0 |
| Fig , dried | 63.9 | 47.9 | 22.9 | 24.8 | 0.9 | 0.93 | 0.15 |
| Grapes | 18.1 | 15.5 | 8.1 | 7.2 | 0.2 | 1.1 | 1 |
| Umbilical orange | 12.5 | 8.5 | 2.25 | 2.0 | 4.3 | 1.1 | 50.4 |
| peach | 9.5 | 8.4 | 1.5 | 2.0 | 4.8 | 0.9 | 56.7 |
| pear | 15.5 | 9.8 | 6.2 | 2.8 | 0.8 | 2.1 | 8.0 |
| pineapple | 13.1 | 9.9 | 2.1 | 1.7 | 6.0 | 1.1 | 60.8 |
| plum | 11.4 | 9.9 | 3.1 | 5.1 | 1.6 | 0.66 | 16.2 |
| vegetables | |||||||
| Beetroot | 9.6 | 6.8 | 0.1 | 0.1 | 6.5 | 1.0 | 96.2 |
| carrot | 9.6 | 4.7 | 0.6 | 0.6 | 3.6 | 1.0 | 77 |
| paprika | 6.0 | 4.2 | 2.3 | 1.9 | 0.0 | 1.2 | 0.0 |
| onion | 7.6 | 5.0 | 2.0 | 2.3 | 0.7 | 0.9 | 14.3 |
| sweet potato | 20.1 | 4.2 | 0.7 | 1.0 | 2.5 | 0.9 | 60.3 |
| yam | 27.9 | 0.5 | traces | traces | traces | - | traces |
| Sugar cane | 13-18 | 0.2-1.0 | 0.2-1.0 | 11-16 | 1.0 | high | |
| sugar beet | 17-18 | 0.1-0.5 | 0.1-0.5 | 16-17 | 1.0 | high | |
| Grain | |||||||
| Corn | 19.0 | 6.2 | 1.9 | 3.4 | 0.9 | 0.61 | 15.0 |
chemistry
Of central importance in carbohydrate chemistry and biochemistry is the glycosidic bond between two monosaccharides or between a monosaccharide and a hydroxyl group , an amino group , a thiol group or a seleno group of another molecule. The cyclic full acetal of a sugar formed in this way is called a glycoside . Carbohydrates are hydroxy aldehydes or hydroxy ketones and compounds derived therefrom. In their open-chain form, they have at least one aldehyde group or keto group in addition to at least two hydroxy groups . If it is a question of a hydroxyaldehyde (carbonyl group on a terminal carbon atom (aldehyde)), it is called an aldose , if it is a hydroxyketone (i.e. with a carbonyl group on an internal carbon atom and hydroxyl groups), the name is sugar as ketosis (synonymous with Ulose ). The carbonyl function is a highly reactive functional group : The easy oxidizability to carboxylic acid , the reduction to alcohol and the easy nucleophilic attack on the carbon atom of the carbonyl group should be mentioned here.
structure
The following table shows some examples of the variety of natural carbohydrate structures. In principle, pentoses and hexoses can form both five and six rings, creating a new center of chirality, so that, including the open-chain form, five isomers already exist for a monosaccharide. Through glycosidic bonds, monosaccharides can combine to form oligo- and polysaccharides. This theoretically expands the number of possible carbohydrate structures to a very large variety.
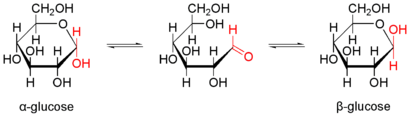
The example of α- D -glucopyranose shows various equivalent forms of presentation.
The pyranoses (ring-shaped monosaccharides with 6 atoms in the ring) adopt a tub or chair conformation. Since the carbon atoms in pyranoses sp 3 -hybridised, they take in a ring shape preferably the energetically more stable chair - conformation and to a lesser extent the trays conformation a.
Oxidation and reduction
By oxidizing agents, aldoses are oxidized to aldonic acids during oxidation at the first carbon atom . As an example, gluconic acid is produced from glucose . Under basic conditions, this applies not only to the aldoses, but also to the ketoses, which are rearranged by the base in a complex reaction (the aldose form occurring in the course of the keto-enol tautomerism is stabilized). When the last carbon atom is oxidized, uronic acids are formed (e.g. glucuronic acid ) and with stronger oxidation the aric acids are formed with two carboxyl groups, a form of dicarboxylic acids , for example glucaric acid is formed from glucose through oxidation up to two carboxyl groups .
If the carbonyl function is reduced to a hydroxyl group, a so-called alditol is obtained .
Cyclization to the hemiacetal and mutarotation
With carbohydrates dissolved in water, a chemical equilibrium of different forms of the respective carbohydrate is established within minutes to hours. An intramolecular nucleophilic attack by one of the hydroxyl groups on the carbonyl carbon atom forms a cyclic hemiacetal , which is usually very favorable in terms of energy. Here, predominantly six-membered rings (pyranose shape) are formed, which have a very low ring tension , but five-membered rings (furanose shape) are also formed to a lesser extent. Other ring sizes do not occur because they have too high a ring tension. A new center of chirality is also created . The two resulting forms are diastereomers and are denoted α and β. In aqueous solution, the α- and β-pyranose and -furanose forms form an equilibrium reaction with one another and with the open-chain form. An aqueous solution of pure α-glucopyranose therefore turns into an equilibrium mixture of α- and β-glucopyranose and furanose (38% α-Glcp, 62% β-Glcp, 0% α-Glcf, 0.14% β -Glcf, 0.002% open-chain). The measurable change in the rotation value is called mutarotation . The proportions of the different forms on the anomeric carbon atom change. While aliphatic aldehydes are gradually oxidized to carboxylic acids by atmospheric oxygen, carbohydrates are considerably less sensitive due to the formation of acetals, which is undoubtedly of enormous importance for such an important class of biomolecules. With glycosidically bound monosaccharides there is no mutarotation.
Amadori products and Maillard reaction
The open-chain aldehyde of the carbohydrate reacts reversibly with amines (e.g. in amino acids, proteins) via an imine to form Amadori products , which in turn can also condense with amines or amino acids and rearrange irreversibly:
This non-enzymatic reaction with amino acids and proteins takes place relatively frequently in the organism and is one of the central processes in aging (e.g. age spots ), as the reaction products cannot be broken down by the body. It also plays an important role in the thermal preparation of foods, e.g. B. frying and cooking. The typical browning occurs because conjugated ring systems are formed that are colored. These products of the so-called Maillard reaction are also decisive for the taste of prepared foods.
synthesis
Various chemical syntheses of carbohydrates have been described. Since there are several hydroxyl groups on a carbohydrate , those that should not react are provided with protective groups .
biochemistry
Mono-, di- and polysaccharides, together with fats and proteins, make up the quantitatively largest usable and non-usable ( fiber ) portion of food . In addition to their central role as energy carriers, they play an important role in biological signaling and recognition processes (e.g. cell contacts , blood groups ), as protection against mechanical stress (e.g. glycosaminoglycans in cartilage tissue ) and, above all in the plant kingdom, as a supporting substance . All cells have a layer of carbohydrates on the outside of their cell membranes , the glycocalyx . Outside the cells of higher eukaryotes lies the extracellular matrix with a high proportion of carbohydrates. The totality of carbohydrates in a cell at a given point in time is called the glycom and depends on the condition of the cell. The science to the study of Glykoms is as glycomics referred. The binding of a protein to a carbohydrate occurs through protein-carbohydrate interactions . Oligo- and polysaccharides are built up from monosaccharides by glycosyltransferases and broken down by glycosidases .
Carbohydrates are not essential because the body can produce them in gluconeogenesis from other food components such as proteins and glycerine using energy. Since the brain in particular is highly dependent on glucose as an energy source and cannot use any fats, the blood sugar level must be kept within narrow limits. Its regulation takes place through the interaction of insulin and glucagon . When there is a lack of carbohydrates, the brain is supplied by ketone bodies , which z. B. makes odor noticeable when dieting through acetone . A completely carbohydrate-free diet was tolerated in animal experiments with chickens. A long-term study on children and young adults with the very low-carbohydrate ketogenic diet also showed no health risks. An independent disease in humans due to the lack of carbohydrates is unknown. The energy content of one gram of carbohydrate is around 17.2 kilojoules (4.1 kilograms of calories ).
function
The monosaccharides are used for the biosynthesis of various molecules. The monosaccharides deoxyribose and ribose are used to produce DNA and RNA , respectively . Glucose, fructose and galactose are used to generate energy. Xylulose and ribulose occur in the pentose phosphate pathway .
Oligosaccharides are often attached to proteins and lipids in the course of glycosylation , which results in glycoproteins or glycolipids . Typical monosaccharides in glycosylations are mannose , galactose, xylose , fucose and amino sugars such as N -acetylglucosamine , N -acetylgalactosamine and neuraminic acid . In addition, the disaccharides sucrose (from glucose and fructose) and lactose (from glucose and galactose) are formed as a source of energy.
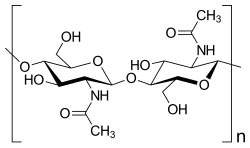
Polysaccharides are either storage forms of monosaccharides for energy metabolism or, as structural carbohydrates, have a structural function for the stability of a cell (cellulose and hemicellulose in plants and many algae , agarose in some algae and chitin in fungi and arthropods ). Glucose polysaccharides are formed from glucose as reserve substances for energy metabolism - glycogen in animals, amylopectin and amylose (components of starch) in plants - or the structural polymers cellulose and hemicellulose. More than 100 different monosaccharides have been described in bacteria, which occur, among other things, in lipopolysaccharides , in polysaccharides of the bacterial capsule or as secreted polysaccharides.
biosynthesis

Simple sugars are built up by plants in the Calvin cycle through photosynthesis from carbon dioxide and water . In practically all living things, these simple sugars are chained to form multiple sugars for storage or for cell construction. Plants synthesize the polysaccharides of starch in the plastids (e.g. chloroplasts ): amylose and amylopectin. Ultimately, almost all biomolecules are formed directly or indirectly through photosynthesis, be it through photosynthesis or, in the case of bacteria, fungi and animals, through the digestion of plant material or through the digestion of herbivores and the subsequent biosynthesis of the biomolecules from the original metabolites of photosynthesis.
Animals also produce monosaccharides, in the form of glucose via gluconeogenesis from other ingested metabolites. They form the long-chain storage polysaccharide glycogen from glucose , especially in the liver and muscles . All other monosaccharides can also be produced from glucose. The energy supply of the brain and the renal medulla depends on the metabolism of glucose as an energy supplier, since fats do not use it directly for energy, but only the keto bodies formed from it in the liver . In starvation situations without carbohydrate intake or with increased muscle work, glucose is therefore synthesized in gluconeogenesis from the metabolic products lactate , certain amino acids ( glucogenic amino acids , including alanine ) and glycerine . Gluconeogenesis uses some enzymes from glycolysis , the breakdown pathway of glucose to produce high-energy ATP and NADH + H + , but it is by no means to be understood as the reverse of these, as a few crucial steps are different and take place exergonically with their own enzymes . Glycolysis and gluconeogenesis are therefore not reversible. Glycolysis and gluconeogenesis are reciprocally regulated; that is, they are almost mutually exclusive in one and the same cell . Different organs can, however, go one way and the other at the same time. For example, when there is strong muscle activity, glycolysis and thus lactate release takes place in the muscle, and gluconeogenesis using lactate in the liver. This shifts part of the metabolic load to the liver, described by the Cori cycle .
admission
In contrast to plants, bacteria, fungi and animals take up carbohydrates. In addition to fat and protein as nutrients, carbohydrates are an essential part of animal nutrition . In humans, 98% of the absorbable carbohydrates (i.e. the non-dietary fibers) are metabolized. Important staple foods that contain a high proportion of carbohydrates are the various types of grain that are processed into food ( rice , wheat , corn , millet , rye , oats ) or used as fodder (especially barley , oats, corn, triticale) ). The starchy cereal products are u. a. Bread , pasta , cakes, etc. v. a. m. The tubers of the potato , a nightshade plant , and the peas , beans and lentils belonging to the legumes also have a high carbohydrate content.
When glucose is absorbed from food, it does not matter how quickly this happens in situations where there is a great need for energy. Since the glucose in food is mostly in a more or less oligomerized or polymerized, more precisely: polycondensed, form, the glucose chains in the digestive tract have to be split up, which happens at different speeds depending on the length of the chains. Are z. If, for example, starchy foods such as bread or potatoes are eaten, the digestive enzymes break down the starch glucose chain into individual fragments and finally down to the individual glucose molecules, which gradually pass into the bloodstream. The carbohydrates are split into monosaccharides by various glycosidases among the digestive enzymes. In eukaryotes there are three groups of transport proteins for monosaccharides: glucose transporters (GLUT), sodium / glucose cotransporters (SGLT) and SWEET . The glycemic index (GI) is a measure of the speed at which the starch is broken down and the glucose components are absorbed .
Carbohydrates for the structural stabilization of cells (structural carbohydrates, e.g. cellulose and chitin) can only be digested to a limited extent by mammals with single-cavity stomachs, but largely or completely by ruminants ( Ruminantia ), camel-like ( Camelidae ) (these are also ruminants, but not in the systematic sense because rumination developed independently in them) and equine species ( Equidae ).
Plant species that primarily contribute to the intake of carbohydrates in human nutrition are compiled in the list of useful plants . Nonabsorbable carbohydrates are fiber. Non-absorbable oligosaccharides increase the water content in the stool and are partially fermented by the intestinal flora . Cellulose, which occurs in large quantities as a supporting substance in the plant world, is indigestible for humans because no cellulase is produced by humans . Cellulose can, however, be used by ruminants such as cattle , sheep and goats , as these make use of the microbial digestion through their intestinal flora in their fore-stomachs ( rumen ) .
The Nutrition Societies in Germany, Austria and Switzerland recommend that carbohydrates contain more than 50% of the calories in food. The European Food Safety Authority (EFSA) recommends 45-60% calories, 45-65% is recommended in the US and the WHO recommends 55-75% calories. The WHO also recommends that no more than 10% of the daily calorie intake should be sugar. Carbohydrates with a high glycemic index, such as sugar, promote excessive intake of calories and hyperphagia . Soluble carbohydrates make you feel less satiated . For long-term vigorous physical activity, an intake of 30 to 60 g per hour is recommended, for more than 2.5 hours an intake of 90 g per hour is recommended.
Dismantling
The direct energy currency for biological processes is adenosine triphosphate (ATP), which, for example, drives muscle contraction and is involved in almost all energy-consuming processes. However, it is only present in the cells in low concentrations and has to be replenished in the cells through aerobic and anaerobic degradation of high-energy compounds such as fats, carbohydrates or proteins. Carbohydrates are the main source of energy for the organism, most often as glucose, followed by fructose, galactose and mannose. These monosaccharides are metabolized in glycolysis. In contrast to fats, they can be used relatively quickly because they provide energy (anaerobically) even when there is a lack of oxygen. Every cell in the body can absorb glucose through the cell membrane . The liver can release them if necessary. In the cells of the various organs, it can either be metabolized to provide the chemical energy for muscle work, anabolic processes or brain activity, or it can be stored as glycogen in the form of glucose chains . The breakdown of glycogen to glucose takes place via glycogenolysis .
The physiological generation of energy from carbohydrates normally takes place in non-oxidative glycolysis and in the presence of oxygen in the oxidative citrate cycle . The oxidation steps in the citrate cycle consist of splitting off hydrogen, which is fed into the respiratory chain by a hydrogen carrier and there is oxidized to water with oxygen. The membrane potential generated on the mitochondrial membrane provides by far the most energy for the synthesis of ATP from ADP . In animals with a lack of oxygen, lactic acid fermentation takes place to lactate and in yeasts alcoholic fermentation to ethanol .
Only when the supply of carbohydrates to the tissues is significantly greater than their consumption is the excess converted into fat and stored as depot fat. Fats have a higher physiological calorific value than carbohydrates because the carbon atoms of lipids have a higher oxidation number . Lipids do not have a hydrate shell , which is why they are more space-saving than carbohydrates for long-term energy storage. In addition, they cause better thermal insulation of the body.
Fat deposits are constantly being used for energy and not only when the glycogen stores in the muscles are reduced. The adenosine triphosphate (ATP) for intense muscle work is supplied by four energy sources. In the first minute of vigorous physical activity, the greatest share of this is due to the utilization of creatine phosphate (from protein metabolism), which has a higher phosphate group transfer potential than ATP and can therefore quickly replenish it. From the second minute onwards, the anaerobic sugar utilization and the oxidative sugar utilization take over. Fat loss accounts for the lowest proportion with around 23% of the energy provided during vigorous physical activity. The more intense the effort, the more the proportion of the first, especially anaerobic, parts increases. As a result, the relative proportion of fat loss decreases with an increased pulse rate, but the absolute amount of utilized fat does increase, as the total energy expenditure also increases. The proportion of the anaerobic metabolism, which increases with vigorous exercise, is related to the decreasing supply of oxygen in the muscle during strong muscle work, since the breakdown of fat in the metabolism is an aerobic process. With low physical exertion such as going for a walk, creatine phosphate is broken down in the first minute, then mainly fat is broken down and only a small part of about 12% of the energy is provided by carbohydrates.
Untrained athletes are often advised to start out persistently and with poor performance (“running without breathing”). However, there is the view that the energy balance alone is decisive in sport, as z. B. after the exertion, a further breakdown of fats takes place. So if a lot of glucose is converted during training, the more fat is broken down in the recovery phase. Other factors affecting fat loss are the type and frequency of food intake, since fat loss is inhibited with each output of insulin. With increasing training, the muscle mass increases, the oxygen uptake improves, resulting in increased fat loss. Competitive athletes train to optimize their glycogen stores before a competition by taking the appropriate time to eat, as this is an important energy store for short-term performance peaks.
In a weight loss diet, lowering your carbohydrate intake is just as effective as lowering your fat intake.
Blood sugar
The body's acute energy supply is essentially guaranteed by the glucose dissolved in the blood. Their concentration in the blood, the blood sugar level , is kept within narrow limits. During digestion, glucose in the small intestine is absorbed from the food pulp as a monosaccharide and released into the blood. The blood sugar level therefore rises after eating. The glucose absorbed into the blood must first be temporarily stored. The signal for this is provided by insulin , a peptide hormone . It signals the muscle and liver tissue to absorb more glucose from the blood and to chain it to glycogen.
fermentation
Fermentation is a metabolic process in which carbohydrates are broken down to generate energy in the absence of oxygen ( anaerobia ). It is mainly used by microorganisms in nature , but plants can fall back on it when there is a lack of oxygen. Lactic acid fermentation takes place in the muscles when there is a lack of oxygen .
Fermentations are used in a variety of ways to produce and refine food. During lactic acid fermentation, lactose is converted into lactic acid and used to make yoghurt , quark and buttermilk . The production of sourdough and silage are based on the fermentation of carbohydrates into lactic acid. In the cheese -Production malolactic fermentation is an important intermediate step.
During alcoholic fermentation , different types of sugar are fermented into alcohol. To be mentioned here u. a. Malt sugar in beer brewing and glucose in vats of wine . Starchy foods such as potatoes, cereals and rice are z. B. processed into schnapps , fruits into fruit waters .
Compared to cellular respiration , fermentation only generates a small amount of energy, since only the substrate chain phosphorylation can be used instead of the citric acid cycle and subsequent respiratory chain .
List of important carbohydrates
- Simple sugars ( monosaccharides )
- Glucose , also dextrose or, more rarely, dextrose
- Mannose , an epimer of glucose
- Fructose , including fruit sugar
- Ribose , part of ribonucleic acid (RNA)
- Deoxyribose , part of deoxyribonucleic acid (DNA)
- Galactose , also called slimy sugar
- Fucose , an L sugar
- Rhamnose , an L sugar
- Double sugar ( disaccharides )
- Triple sugar ( trisaccharides )
- Melezitose , trisaccharide and the like a. in honey
- Raffinose remains in the
- Umbelliferosis
-
Starch , an important food ingredient
- Amylose structure of starch
- Amylopectin structure of starch
- Agarose , supporting substance of some algae
- Cellulose , a supporting substance in the plant kingdom
- Glycogen , energy store in muscles and liver
- Hyaluronic acid , protection against mechanical stress in the cartilage tissue
- Chitin , supporting substance of the exoskeleton of articulated animals
- Callose , repair carbohydrate in plants
- Fructans , another storage carbohydrate in plants
- Dextrans , bacterial polysaccharide used in industry
- Pectins are a type of dietary fiber
- α-cyclodextrin (ring of six glucose units)
- β-cyclodextrin (seven glucose units)
- γ-cyclodextrin (eight glucose units)
Pathobiochemistry
Excessive intake of carbohydrates, especially sugars, is associated with an increased risk of obesity , metabolic syndrome and type 2 diabetes mellitus . By consuming low-glycemic index carbohydrates, fiber, fats with unsaturated fatty acids, and low-fat protein sources, as well as reducing the intake of sugars and high-glycemic index polysaccharides, the risk of type 2 diabetes can be reduced. Ingesting sugars can cause tooth decay . It has been suggested that carbohydrates with a high glycemic index promote the development of cardiovascular diseases and type 2 diabetes mellitus and that carbohydrates with a low glycemic index protect against them. The protective effect of carbohydrates with a low glycemic index against cardiovascular diseases could not be confirmed. The disorders of the carbohydrate metabolism include the glycogen storage disease Von Gierke disease , mellituria such as fructosuria , galactosemia and diabetes renalis .
Analytics
Carbohydrates are isolated by chromatography or gel electrophoresis , including size exclusion chromatography and capillary electrophoresis . With lectins one can affinity chromatography are performed.
In concentrated solutions of sugars with a small proportion of other carbohydrates, their concentration can be determined with a polarimeter . In the case of sugar mixtures, the concentration can be determined with a refractometer , for example when determining Oechsle during the production of wine, when determining the original wort when brewing beer and as a beekeeping device to determine the water content of honey .
Classic evidence
A general detection of carbohydrates can be carried out by the Molisch sample , the Barfoed sample and the PAS reaction . The distinction between monosaccharides and di-, oligo- or polysaccharides is possible with the Barfoed sample. Aldoses and ketoses can be differentiated by the Selivanov test with resorcinol . Reducing sugars can be detected with the Fehling's test , which forms red-brown copper (I) oxide in the presence of aldehydes and reducing sugars (aldoses and acyloins ). In addition to the Fehling's test, reducing sugars can also be detected with the help of the Benedict reagent (through the color of the precipitated product), with Nylander's reagent , with 3,5-dinitrosalicylic acid or by means of the discoloration of a potassium permanganate solution. The distinction between pentoses and hexoses can be made using the Mejbaum sample with orcin or the bial sample (also with orcin). Diphenylamine can be used to detect deoxyribose using the Dische sample . Acetylated amino sugars can be detected by the Morgan-Elson reaction . Furans are formed under basic conditions , which are then reacted with Ehrlich's reagent . Starch and chitin can be colored with Lugol's solution . Starch can also be stained with Melzer's reagent .
Modern methods
Recent analytical methods for the qualitative and quantitative detection of individual carbohydrates in different specimens used after adequate sample preparation and optionally derivatization , chromatographic separation processes in connection with mass spectrometry . Capillary electrophoresis is also used before mass spectrometry. Selected TMS derivatives are also used for special carbohydrates. Certain carbohydrates can be detected with marked lectins. Different carbohydrates can also be examined using FT - infrared spectroscopy , FT - Raman spectroscopy and nuclear magnetic resonance spectroscopy .
Industrial production and use

Carbohydrates for food are mostly obtained from agriculturally produced grain . Carbohydrates are a renewable resource . Starch is a key ingredient in flour and farinaceous foods. The short-chain carbohydrates (sugar) are used as sweeteners . In purified form, monosaccharides such as glucose syrup or isoglucose are produced from starch and used as sweeteners in the production of food. Sucrose is also used as a sweetener. Purified polysaccharides are, for example, corn starch , wheat starch , potato starch and cellulose . From starch also is starch paste made.
Cellulose from the cotton fiber from the flax fiber and various other plant natural fibers are made from fibrous plants isolated and for the production of textiles used. Cellulose is also used as a raw material for paper and cardboard as well as for the production of biofuels such as cellulosic ethanol . Textiles are also made from converted celluloses such as viscose , modal , lyocell and cupro . Celluloid and cellophane are derivatives of cellulose. Due to the large number of hydroxyl groups, some derivatives of cellulose are used as adhesives , for example in pastes such as methyl cellulose , or as varnishes , such as collodion wool in nitro lacquers .
Various carbohydrates and their derivatives are used as medicinal substances in medicine . For example, glucose is used for infusion solutions . Some carbohydrates are used as raw materials in the manufacture of cytostatics and antibiotics . Various anticoagulants also have a carbohydrate structure, such as heparin .
In biochemistry are polymeric carbohydrates including as filter paper and as a stationary phase in chromatography ( diethylaminoethyl cellulose , carboxymethyl cellulose , cross-linked dextran , cross-linked agarose ) and in the immunoprecipitation used. Nitrocellulose is used for blotting membranes in blotting .
literature
- Thisbe K. Lindhorst : Structure and function of carbohydrates. In: Chemistry in Our Time . Volume 34, 2000, No. 1, pp. 38-52. doi : 10.1002 / 1521-3781 (200002) 34: 1 <38 :: AID-CIUZ38> 3.0.CO; 2-L
- Thomas K. Ritter, Chi-Huey Wong : Carbohydrates in Antibiotic Research - A New Approach to Combating Resistance. In: Angewandte Chemie . Volume 113, No. 19, 2001, pp. 3616-3641. doi : 10.1002 / 1521-3757 (20011001) 113: 19 <3616 :: AID-ANGE3616> 3.0.CO; 2-B
- Robert V. Stick, Spencer Williams: Carbohydrates: The Essential Molecules of Life . 2nd edition, Elsevier, 2010. ISBN 978-0-08-092702-2 .
- Jochen Lehmann: Carbohydrates. Chemistry and biology. Thieme, Stuttgart / New York 1996, ISBN 3-13-532902-X .
Web links
Individual evidence
- ↑ Frieder W. Lichtenthaler: Carbohydrates , in: Ullmann's Encyclopedia of Industrial Chemistry , 7th edition, Wiley-VCH, 2011, ISBN 978-3-527-32943-4 .
- ^ A b c Donald Voet, Judith G. Voet: Biochemistry, 4th Edition. John Wiley & Sons, 2010, ISBN 978-0-470-57095-1 . P. 359.
- ^ Annals of Chemistry. 51, 30, 1844.
- ↑ P. Avenas: Etymology of main polysaccharide names . In: P. Navard (Ed.): The European Polysaccharide Network of Excellence (EPNOE) . Springer-Verlag, Vienna 2012.
- ^ Schweiger's Journal for Chemistry and Physics. 14, 389, 1814.
- ↑ Annales de chimie et des physique (2) 12, 172, 1819.
- ↑ Phil. Tr. 1827, 355.
- ^ Emil Fischer - Facts. In: nobelprize.org. July 15, 1919, accessed July 15, 2018 .
- ↑ Otto Meyerhof - Facts. In: nobelprize.org. July 15, 2018, accessed July 15, 2018 .
- ↑ Hans von Euler-Chelpin - Facts. In: nobelprize.org. Accessed September 3, 2018 .
- ^ Arthur Harden - Facts. In: nobelprize.org. June 17, 1940, accessed September 3, 2018 .
- ↑ Bernardo Houssay - Facts. In: nobelprize.org. September 21, 1971, accessed July 15, 2018 .
- ^ Carl Cori - Facts. In: nobelprize.org. October 20, 1984, accessed July 15, 2018 .
- ^ Gerty Cori - Facts. In: nobelprize.org. October 26, 1957, accessed July 15, 2018 .
- ↑ Luis Leloir - Facts. In: nobelprize.org. July 15, 2018, accessed July 15, 2018 .
- ↑ U. Satyanarayana: Biochemistry. Elsevier Health Sciences, 2014, ISBN 978-8-131-23713-7 . P. 9.
- ↑ IUPAC: Entry on molecular entity . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C00820 .
- ↑ a b c d H.-D. Belitz, W. Grosch, P. Schieberle: Food Chemistry. Springer Science & Business Media, 2009, ISBN 978-3-540-69933-0 , pp. 248, 262.
- ↑ Waldemar Ternes , Alfred Täufel, Lieselotte Tunger, Martin Zobel (eds.): Food Lexicon . 4th, comprehensively revised edition. Behr, Hamburg 2005, ISBN 3-89947-165-2 . , Pp. 1606-1612.
- ↑ a b c d e f g h Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Stryer Biochemistry. 7th edition, Springer-Verlag, 2010, ISBN 978-1-4292-2936-4 , pp. 319-321.
- ↑ a b c d e Steve W. Cui: Food Carbohydrates. CRC Press, 2005, ISBN 978-0-203-48528-6 , pp. 6, 7.
- ↑ a b c d e B. Sivasankar: Food Processing and Preservation. PHI Learning Pvt. Ltd., 2002, ISBN 978-8-120-32086-4 , p. 23.
- ↑ a b Kenji Kamide: Cellulose and Cellulose Derivatives. Elsevier, 2005, ISBN 978-0-08-045444-3 , p. 1.
- ^ Search the USDA National Nutrient Database for Standard Reference. Nal.usda.gov, accessed December 10, 2014 .
- ↑ IUPAC Nomenclature of Carbohydrates or Section 2-Carb-33: Glycosides and glycosyl compounds .
- ^ A b Reginald H. Garrett: Biochemistry. Cengage Learning, 2012, ISBN 978-1-133-10629-6 . Pp. 194, 199.
- ^ A b c d Donald Voet, Judith G. Voet: Biochemistry, 4th Edition. John Wiley & Sons, 2010, ISBN 978-0-470-57095-1 . P. 383.
- ↑ Michael Sinnott: Carbohydrate Chemistry and Biochemistry. Royal Society of Chemistry, 2007, ISBN 978-0-85404-256-2 , p. 16.
- ^ Hans-Dieter Belitz , Werner Grosch, Peter Schieberle : Food chemistry . Springer, Berlin 2009. ISBN 978-3-540-69935-4 . Pp. 270-289.
- ↑ a b D. B. Werz: Chemical synthesis of carbohydrates and their surface immobilization: a brief introduction. In: Methods in molecular biology. Volume 808, 2012, pp. 13-29, doi : 10.1007 / 978-1-61779-373-8_2 , PMID 22057515 .
- ↑ R. Renner, AM Elcombe: Metabolic effects of feeding "carbohydrate-free" diets to chicks. In: The journal of nutrition. Volume 93, 1967, pp. 31-36.
- ↑ Johns Hopkins Medical Institutions: High-Fat Ketogenic Diet to Control Seizures Is Safe Over Long Term, Study Suggests. Science Daily, February 17, 2010, accessed on March 4, 2011 (English): “ Despite its temporary side effects, we have always suspected that the ketogenic diet is relatively safe long term, and we now have proof. "
- ↑ Eric C Westman: Is dietary carbohydrate essential for human nutrition? In: Am J Clin Nutr . tape 75 , no. 5 . American Society for Clinical Nutrition, May 1, 2002, pp. 951–953 ( ajcn.org [accessed April 25, 2011]). Is dietary carbohydrate essential for human nutrition? ( Memento of the original from September 21, 2010 in the Internet Archive ) Info: The archive link was inserted automatically and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Entry on carbohydrates. In: Römpp Online . Georg Thieme Verlag, accessed on 2014-06-20.
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Stryer Biochemistry. 7th edition, Springer-Verlag, 2010, ISBN 978-1-4292-2936-4 , pp. 329-337.
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Stryer Biochemistry. 7th edition, Springer-Verlag, 2010, ISBN 978-1-4292-2936-4 , p. 349.
- ↑ a b G. Widmalm: A perspective on the primary and three-dimensional structures of carbohydrates. In: Carbohydrate research. Volume 378, August 2013, pp. 123-132, doi : 10.1016 / j.carres.2013.02.005 , PMID 23522728 .
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Stryer Biochemistry. 7th edition, Springer-Verlag, 2010, ISBN 978-1-4292-2936-4 , pp. 479-491.
- ^ Peter C. Heinrich: Löffler / Petrides Biochemistry and Pathobiochemistry. Springer-Verlag, 2014, ISBN 978-3-642-17972-3 , p. 27.
- ↑ Florian Horn: Biochemistry of humans. Georg Thieme Verlag, 2009, ISBN 978-3-13-130884-9 , pp. 146-149.
- ↑ Jan Koolman: Pocket Atlas of Biochemistry. Georg Thieme Verlag, 2003, ISBN 978-3-13-759403-1 , p. 180.
- ^ Reginald H. Garrett: Biochemistry. Cengage Learning, 2016, ISBN 978-1-305-88689-6 , p. 757.
- ^ Vassilis Mougios: Exercise Biochemistry. Human Kinetics, 2006, ISBN 978-0-7360-5638-0 , p. 170.
- ^ Norman N. Potter: Food Science. Springer Science & Business Media, 2012, ISBN 978-1-4615-4985-7 , p. 47.
- ↑ LQ Chen, LS Cheung, L. Feng, W. Tanner, WB Frommer: Transport of sugars. In: Annual review of biochemistry. Volume 84, 2015, pp. 865-894, doi : 10.1146 / annurev-biochem-060614-033904 , PMID 25747398 .
- ^ SJ Shepherd, MC Lomer, PR Gibson: Short-chain carbohydrates and functional gastrointestinal disorders. In: The American Journal of Gastroenterology. Volume 108, number 5, May 2013, pp. 707-717, doi : 10.1038 / ajg.2013.96 , PMID 23588241 .
- ↑ a b A. E. Buyken, DJ Mela, P. Dussort, IT Johnson, IA Macdonald, JD Stowell, FJ Brouns: Dietary carbohydrates: a review of international recommendations and the methods used to derive them. In: European Journal of Clinical Nutrition. [Electronic publication before printing] April 2018, doi : 10.1038 / s41430-017-0035-4 , PMID 29572552 .
- ↑ WHO : Joint FAO / WHO Scientific update on carbohydrates in human nutrition . In: European Journal of Clinical Nutrition , Volume 61 (Supplement 1), December 2007.
- ↑ B. Lennerz, JK Lennerz: Food Addiction, High-Glycemic-Index Carbohydrates, and Obesity. In: Clinical chemistry. Volume 64, number 1, January 2018, pp. 64-71, doi : 10.1373 / clinchem.2017.273532 , PMID 29158252 , PMC 5912158 (free full text).
- ^ A. Bosy-Westphal, MJ Müller: Impact of carbohydrates on weight regain. In: Current opinion in clinical nutrition and metabolic care. Volume 18, Number 4, July 2015, pp. 389-394, doi : 10.1097 / MCO.0000000000000193 , PMID 26049636 .
- ^ A. Pan, FB Hu: Effects of carbohydrates on satiety: differences between liquid and solid food. In: Current opinion in clinical nutrition and metabolic care. Volume 14, Number 4, July 2011, pp. 385-390, doi : 10.1097 / MCO.0b013e328346df36 , PMID 21519237 .
- ↑ LM Burke, JA Hawley, SH Wong, AE Jeukendrup: Carbohydrates for training and competition. In: Journal of sports sciences. Volume 29 Suppl 1, 2011, pp. S17-S27, doi : 10.1080 / 02640414.2011.585473 , PMID 21660838 .
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Stryer Biochemistry. 7th edition, Springer-Verlag, 2010, ISBN 978-1-4292-2936-4 , p. 430.
- ^ A b Donald Voet, Judith G. Voet: Biochemistry, 4th Edition. John Wiley & Sons, 2010, ISBN 978-0-470-57095-1 .
- ↑ Donald Voet, Judith G. Voet: Biochemistry, 4th Edition. John Wiley & Sons, 2010, ISBN 978-0-470-57095-1 . P. 790.
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Stryer Biochemistry. 7th edition, Springer-Verlag, 2010, ISBN 978-1-4292-2936-4 , p. 499.
- ↑ KJ Acheson, Y Schutz, T Bessard, K Anantharaman, JP Flatt: Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man . In: The American Journal of Clinical Nutrition . tape 48 , no. 2 , August 1, 1988, ISSN 0002-9165 , pp. 240–247 , doi : 10.1093 / ajcn / 48.2.240 ( oup.com [accessed December 12, 2019]).
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer: Stryer Biochemistry. 7th edition, Springer-Verlag, 2010, ISBN 978-1-4292-2936-4 , p. 436.
- ^ A b c Donald MacLaren: Biochemistry for Sport and Exercise Metabolism. John Wiley & Sons, 2011, ISBN 978-1-119-96782-8 , p. 7.
-
↑ Ingo Froböse : “Running in the morning before breakfast burns more fat!” And: “After muscle training you should stretch!” ( Memento from August 13, 2009 in the Internet Archive )
The biggest mistakes in sports . Episode 1. In: ARD morning magazine . - ↑ Kurt A. Moosburger (specialist in internal medicine and sports doctor, Institute for Biostatistics and Documentation, University of Innsbruck): "Fat burning" in sport: myth and truth . ( Memento of the original from February 2, 2010 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF). In: Healthier Living. 05/2000.
- ^ ME Thompson, MB Noel: Issues in Nutrition: Carbohydrates. In: FP essentials. Volume 452, January 2017, pp. 26-30, PMID 28092151 .
- ↑ AY Tamime: yogurt. Woodhead Publishing, 1999, ISBN 978-1-85573-399-2 , p. 11.
- ↑ Cecie Starr: Biology: Concepts and Applications. Cengage Learning, 2014, ISBN 978-1-285-42781-2 . P. 123.
- ↑ Carlos Ricardo Soccol: Fermentation Processes Engineering in the Food Industry. CRC Press, 2013, ISBN 978-1-4398-8768-4 , pp. 60 ff.
- ↑ Michael J. Lewis: Brewing. Springer Science & Business Media, 2012, ISBN 978-1-4615-0729-1 , p. 319.
- ↑ Eduardo Pires: Biochemistry of Beer Fermentation. Springer, 2015, ISBN 978-3-319-15189-2 , p. 11.
- ↑ M. Victoria Moreno-Arribas: Wine Chemistry and Biochemistry. Springer Science & Business Media, 2008, ISBN 978-0-387-74118-5 , p. 655.
- ^ RJ Johnson, T. Nakagawa, LG Sanchez-Lozada, M. Shafiu, S. Sundaram, M. Le, T. Ishimoto, YY Sautin, MA Lanaspa: Sugar, uric acid, and the etiology of diabetes and obesity. In: Diabetes. Volume 62, number 10, October 2013, pp. 3307–3315, doi : 10.2337 / db12-1814 , PMID 24065788 , PMC 3781481 (free full text).
- ^ A b D. C. Greenwood, DE Threapleton, CE Evans, CL Cleghorn, C. Nykjaer, C. Woodhead, VJ Burley: Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. In: Diabetes care. Volume 36, number 12, December 2013, pp. 4166-4171, doi : 10.2337 / dc13-0325 , PMID 24265366 , PMC 3836142 (free full text).
- ↑ KC Maki, AK Phillips: Dietary substitutions for refined carbohydrate that show promise for reducing risk of type 2 diabetes in men and women. In: The Journal of Nutrition. Volume 145, number 1, January 2015, pp. 159S-163S, doi : 10.3945 / jn.114.195149 , PMID 25527674 .
- ↑ SW Rizkalla: Glycemic index: is it a predictor of metabolic and vascular disorders? In: Current opinion in clinical nutrition and metabolic care. Volume 17, Number 4, July 2014, pp. 373-378, doi : 10.1097 / MCO.0000000000000070 , PMID 24878873 .
- ↑ C. Clar, L. Al-Khudairy, E. Loveman, SA Kelly, L. Hartley, N. Flowers, R. Germanò, G. Frost, K. Rees: Low glycaemic index diets for the prevention of cardiovascular disease. In: The Cochrane database of systematic reviews. Volume 7, 07 2017, S. CD004467, doi : 10.1002 / 14651858.CD004467.pub3 , PMID 28759107 .
- ↑ Ludwig Weissbecker: Diseases of the carbohydrate metabolism. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition, ibid. 1961, pp. 1117–1119.
- ^ S. Honda: Separation of neutral carbohydrates by capillary electrophoresis. In: Journal of chromatography. A. Volume 720, Number 1-2, January 1996, pp. 337-351, PMID 8601200 .
- ↑ Hans Molisch, in: Monatsh. Chem. (1886), Vol. 7, p. 198.
- ↑ C. Barfoed: About the detection of grape sugar in addition to dextrin and related bodies . In: Journal for Analytical Chemistry . 12, No. 1, 1873, p. 27. doi : 10.1007 / BF01462957 .
- ↑ Theodor Seliwanoff: "Note on a fruit sugar reaction". In: Reports of the German Chemical Society , 20 (1), 1887, pp. 181-182, doi : 10.1002 / cber.18870200144 .
- ↑ H. Fehling: Quantitative determination of sugar in urine. In: Archives for Physiological Medicine (1848), Volume 7, pp. 64–73.
- ^ Stanley Benedict: A reagent for the detection of reducing sugars . In: J. Biol. Chem. , 1909, 5, pp. 485-487.
- ^ Emil Nylander: About alkaline bismuth solution as a reagent for glucose in urine , magazine for physiological chemistry . Volume 8, Issue 3, 1884, pp. 175-185 abstract
- ↑ JB Sumner: Dinitrosalicylic acid: a reagent for the estimation of sugar in normal and diabetic urine. In: Journal of Biological Chemistry (1921), Volume 47, p. 5.
- ↑ Wanda Mejbaum: About the determination of small amounts of pentose, especially in derivatives of adenylic acid. In: Hoppe-Seyler's magazine for physiological chemistry. 258, 1939, p. 117, doi : 10.1515 / bchm2.1939.258.2-3.117 .
- ↑ M. Bial: About the diagnosis of pentosuria with the reagent I have indicated. In: DMW - German Medical Weekly. 29, 1903, p. 477, doi : 10.1055 / s-0028-1138555 .
- ↑ L. Jaenicke: memory image Zacharias Dische: Jack of all trades, in the memory through obsolete reaction and current realization. In: Biospectrum . Volume 1, pp. 106-108. biospektrum.de (PDF).
- ↑ Michael Sinnott: Carbohydrate Chemistry and Biochemistry. Royal Society of Chemistry, 2013, ISBN 978-1-78262-632-9 . Pp. 721, 722.
- ^ MV Melzer: L'ornementation des spores de Russules . In: Bull. Soc. myc. Fr. (1924). Volume 40, pp. 78-81.
- ^ AI Ruiz-Matute, O. Hernández-Hernández, S. Rodríguez-Sánchez, ML Sanz, I. Martínez-Castro: Derivatization of carbohydrates for GC and GC-MS analyzes. In: Journal of Chromatography B . Volume 879, number 17-18, May 2011, pp. 1226-1240, doi : 10.1016 / j.jchromb.2010.11.013 , PMID 21186143 .
- ↑ B. Zhu, F. Liu, X. Li, Y. Wang, X. Gu, J. Dai, G. Wang, Y. Cheng, C. Yan: Fast quantification of endogenous carbohydrates in plasma using hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry. In: J Sep Sci . 38 (1), Jan 2015, pp. 34-41. PMID 25359182 .
- Jump up ↑ B. Du, L. Wu, X. Xue, L. Chen, Y. Li, J. Zhao, W. Cao: Rapid Screening of Multiclass Syrup Adulterants in Honey by Ultrahigh-Performance Liquid Chromatography / Quadrupole Time of Flight Mass Spectrometry . In: J Agric Food Chem . 63 (29), Jul 29, 2015, pp. 6614-6623. PMID 26151590 .
- ↑ DJ Harvey: Derivatization of carbohydrates for analysis by chromatography; electrophoresis and mass spectrometry. In: J Chromatogr B Analyt Technol Biomed Life Sci . 879 (17-18), May 15, 2011, pp. 1196-1225, Review. PMID 21145794 .
- ↑ MB Clarke, DZ Bezabeh, CT Howard: Determination of carbohydrates in tobacco products by liquid chromatography-mass spectrometry / mass spectrometry: a comparison with ion chromatography and application to product discrimination. In: J Agric Food Chem . 54 (6), Mar 22, 2006, pp. 1975-1981. PMID 16536564 .
- ↑ DJ Harvey: Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption / ionization mass spectrometry: An update for 2013–2014. In: Mass spectrometry reviews. Volume 37, number 4, July 2018, pp. 353-491, doi : 10.1002 / mas.21530 , PMID 29687922 .
- ↑ J. Zaia: Capillary electrophoresis-mass spectrometry of carbohydrates. In: Methods in molecular biology. Volume 984, 2013, pp. 13-25, doi : 10.1007 / 978-1-62703-296-4_2 , PMID 23386333 , PMC 4108199 (free full text).
- ↑ I. Boldizsár, Z. Füzfai, I. Molnár-Perl: Characteristic fragmentation patterns of trimethylsilyl and trimethylsilyl-oxime derivatives of plant disaccharides as obtained by gas chromatography coupled to ion-trap mass spectrometry. In: J Chromatogr A . 1218 (43), Oct 28, 2011, pp. 7864-7848. PMID 21924428 .
- ↑ V. Ratsimba, JM Fernández García, J. Defaye, H. Nigay, A. Voilley: Qualitative and quantitative evaluation of mono- and disaccharides in D-fructose, D-glucose and sucrose caramels by gas-liquid chromatography-mass spectrometry. Di-D-fructose dianhydrides as tracers of caramel authenticity. In: J Chromatogr A . 844 (1-2), Jun 4, 1999, pp. 283-293. PMID 10399331 .
- ↑ E. Wiercigroch, E. Szafraniec, K. Czamara, MZ Pacia, K. Majzner, K. Kochan A. Kaczor, M. Baranska, K. Malek: Raman and infrared spectroscopy of carbohydrates: A review. In: Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy. Volume 185, October 2017, pp. 317-335, doi : 10.1016 / j.saa.2017.05.045 , PMID 28599236 .
- ^ SZ Dziedzic: Handbook of Starch Hydrolysis Products and their Derivatives. Springer Science & Business Media, 2012, ISBN 978-1-4615-2159-4 . P. 2, 56.
- ↑ a b Kurt Schönburg: Coating Techniques Today. Beuth Verlag, 2013, ISBN 978-3-410-21090-0 , p. 114.
- ^ John N. Owens: Forests And Forest Plants - Volume II. EOLSS Publications, 2009, ISBN 978-1-905839-39-1 , p. 159.
- ↑ Ram B. Gupta: Gasoline, Diesel, and Ethanol Biofuels from Grasses and Plants. Cambridge University Press, 2010, ISBN 978-1-139-48906-5 , pp. 84, 85.
- ↑ Dipa Ray: biocomposites for High-Performance Applications. Woodhead Publishing, 2017, ISBN 978-0-08-100794-5 , p. 23.
- ↑ Bernhard Schrader: Short textbook of organic chemistry. Walter de Gruyter, 2009, ISBN 978-3-11-020360-8 , p. 206.
- ^ WHO Model List of Essential Medicines. In: World Health Organization. October 2013, archived from the original on April 23, 2014 ; Retrieved April 22, 2014 .
- ↑ CAA van Boeckel: Some recent applications of carbohydrates and their derivatives in the pharmaceutical industry. In: Recueil des Travaux Chimiques des Pays-Bas (1986). Volume 105, Issue 2, pp. 35-53. doi: 10.1002 / recl.19861050202 .
- ↑ Berit S. Paulsen: Bioactive Carbohydrate Polymers. Springer Science & Business Media, 1999, ISBN 978-0-7923-6119-0 , p. 47.
- ↑ David Sheehan: Physical Biochemistry. John Wiley & Sons, 2013, ISBN 978-1-118-68748-2 . P. 21.
- ↑ G. Tripathi: Cellular and Biochemical Science. IK International Pvt Ltd, 2010, ISBN 978-8-188-23785-2 , p. 1191.