Callose
Callose (also called callose) is a polysaccharide that is used by plant cells as a universal sealing material in a wide variety of situations.
construction
Callose is a vegetable polysaccharide. β-D-glucose monomers are linked via β-1 → 3-glycosidic bonds to form a polymer or glucan , which in some cases can also have β-1,6 branches. This form of linkage results in a long, helical chain of glucan, which is always in compact form.
Callose can be detected in the plant tissue by staining with the dye aniline blue . This is embedded in the helical structure of the callose and can be detected by fluorescence under UV light . The general formula is (C 6 H 10 O 5 ) n.
synthesis
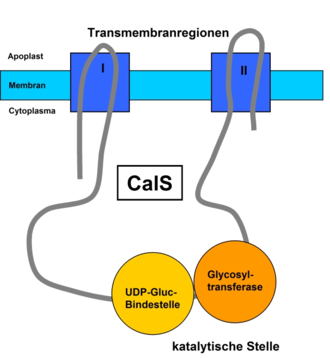
Contrary to the original assumption, the synthesis of callose is not carried out by the rosette-shaped cellulose synthase , but by a membrane-based callose synthase (CalS) ( EC 2.4.1.34 ). The CalS catalyzes a so-called vectorial reaction in which the substrate (UDP-glucose) is bound to the cytoplasmic side of the plasma membrane and, after being linked to the outside of the cell membrane , is deposited in the apoplast , i.e. between the membrane and the cell wall.
CalS is encoded by a family of glucan synthase-like genes (GSL), which has a strong homology to genes involved in glucan biosynthesis in yeasts . The primary sequence of the catalytic subunits of CalS (2000 amino acids ) and cellulose synthase (1000 amino acids) are a clear indication of the separate synthesis of callose. In Arabidopsis thaliana , twelve GSL genes encoding the subunits have been identified so far, which have been named CalS1 to CalS12. In Arabidopsis, these twelve genes are distributed across the five existing chromosomes .
The CalS is present as a protein with several transmembrane domains , which are grouped into two regions. These two regions are connected to the cytoplasm by a hydrophilic loop that contains the catalytic subunit, e.g. B. CalS1 contains. The catalytic site can be divided into UDP-glucose-binding and glycosyltransferase domains. Associated with this hydrophilic loop are also various other proteins that support the synthesis and regulation of the enzyme complex. Sucrose synthase, which generates UDP-glucose by cleaving sucrose, plays a decisive role . Furthermore, according to one hypothesis, an annexin-like membrane protein , a Rop protein , a proline-rich domain, a glycosylation site and two phosphorylation sites can also be found here.
Functions
Callose serves as a universal sealing material for plant cells and is used both in regular development and growth processes and as a reaction to biotic and abiotic stress factors. If there is a need for the cell, callose can be synthesized quickly in large quantities, on the other hand, rapid degradation is also possible.
Typical functions of the callose "in normal operation" of the plant are e.g. B. the appearance in the cell plate of the dividing cell during cytokinesis . In pollen mother cells , the callose is the main component. Even in growing pollen tubes , the advancing protoplast seals the abandoned section of the tube with callose plugs. Callose also closes the pores of the sieve plates of dormant phloem (hibernation) or of defective phloem and thus separates efficiently from the rest of the tissue. In plasmodesmata (cell-cell connection), the callose is a structural component that can regulate the diameter of the canal by means of a jacket around the neck region and thus limit the intercellular transport.
The closure of sieve pores and also the regulation of the size of plasmodesmata are functions that also come into play in stressful situations. So callose prevents u. a. the further spread of viruses via the plasmodesmata. In the event of attacks by fungal hyphae or wounds, callose is very quickly synthesized by the plant as the first physical barrier (papillae) or to close wounds. Such papillae, which in addition to callose also consist of lignin , other polysaccharides, phenolic components, RO intermediates and proteins, are produced by resistant plants immediately after the pathogen has been recognized and deposited at the penetration sites. In this way, they should prevent the impending pathogen entry or at least ensure that the pathogen is slowed down or demobilized. In this way, the attacked cell is given a delay in any case, which gives it time to initiate further defense measures such as the oxidative burst or the use of antipathogenic substances such as PR proteins (pathogen-related protein, pathogen cell wall-degrading enzymes). or phytoalexins . If the cell does enter the pathogen or is infected, it is sealed off with the help of further callose deposits on the plasmodesmata. This isolation leads to the accumulation of carbohydrates and via the so-called sugar sensing to the activation of the sink metabolism (Scharte, Schön & Weis, 2005; Schön 2005). The cell metabolism, which is now set on defense, starts a whole range of further measures, which can end in programmed cell death (PCD: programmed cell death).
regulation
The signaling pathways involved in the regulation of CalS are still largely unknown. However, an important role is ascribed to Ca 2+ ions. For example, in resistant plants, immediately after the start of a pathogen attack in the cells, elicitor- dependent membrane channels open , which triggers an influx of Ca 2+ ions and, at the same time, H + ions. The increase in the Ca 2+ concentration presumably stimulates callose synthesis via the annexin-like membrane protein mentioned above.
A Ca 2+ -independent isoform of callose synthase appears to be involved in the formation of the cell plate. Activation here presumably takes place via a phragmoplastin-Rop interaction (Rop protein on the hydrophilic loop).
In addition, z. B. G proteins , glycosylation (glycosylation site), phosphorylation (phosphorylation sites), the influence of phytohormones (e.g. abscisic acid ) and the achievement of a pH optimum for CalS are discussed as regulatory mechanisms.
See also
literature
- McCurdy, DW. et al . (2008): Wall ingrowth formation in transfer cells: novel examples of localized wall deposition in plant cells . In: Curr Opin Plant Biol 11 (6); 653-661; PMID 18849189 ; doi: 10.1016 / j.pbi.2008.08.005
- Will, T. and van Bel, AJ. (2006): Physical and chemical interactions between aphids and plants . In: J Exp Bot 57 (4); 729-737; PMID 16473888 ; PDF (free full text access)
- Maor, R. and Shirasu, K. (2005): The arms race continues: battle strategies between plants and fungal pathogens . In: Curr Opin Microbiol 8 (4); 399-404; PMID 15996507 ; doi: 10.1016 / j.mib.2005.06.008
- Verma, DP. and Hong, Z. (2001): Plant callose synthase complexes . In: Plant Molecular Biology 47 (6): 693-701; PMID 11785931 ; doi: 10.1023 / A: 1013679111111
- Hans W. Heldt and Birgit Piechulla: Plant biochemistry . Spectrum Academic Publishing House; 4th edition 2008; ISBN 978-3-8274-1961-3 ; P. 258f.
- Sitte P. et al . (2002): Strasburger - Textbook of Botany. Spectrum Academic Publishing House. Heidelberg. Berlin. Oxford. 35th edition.