Sugar as a renewable raw material

Biomass consists mainly of sugar or sugar polymers . Sugar is therefore of great importance as a renewable raw material . It is obtained primarily as the disaccharide sucrose from sugar cane or sugar beet . The sugar polymer starch (a polysaccharide ) consists of the monomer glucose (a monosaccharide ) and is obtained from grain, corn and starch potatoes , for example . Another common glucose polymer is cellulose , which is mainly obtained from wood. An important use is the energy recovery, such as the production of bioethanol and other biofuels from sugar or starch or the thermal use of cellulose as a component of firewood. The material use of sugar is also of great importance. On the one hand, they are used in biotechnology as a source of energy and carbon in fermentation approaches for the production of organic solvents, various raw materials (e.g. for the production of bioplastics ) and others. In chemical processes, sugar is used as a raw material for the production of surfactants , polyols and other products.
Chemistry and manufacturing

Sugars are made up of carbon , oxygen, and hydrogen and are also known as carbohydrates. 3 to 7 carbon atoms form the backbone of a simple sugar ( triose , tetrose , pentose , hexose , heptose ). In the linear sugar molecule, all carbon atoms except one have a hydroxyl group (-OH) and at least one hydrogen atom. The remaining carbon atom has an oxidized hydroxyl group with a carbonyl group. With larger sugar molecules (from pentose) the typical ring structure ( hemiacetal structure) can be formed via this functional group .
The simple sugars, in turn, can be linked to one another in a variety of ways via different glycosidic bonds (α or β) between different carbon atoms of a sugar (in starch, e.g. between carbon atoms 1 and 4).
Numerous different simple and multiple sugars are thus conceivable. Most of them are rare in nature, but a few are common. In addition, the formose process can also be used to extract sugar mixtures based on fossil raw materials.
Sugar can be biotechnologically and chemically converted into a wide variety of products. The chemical reactions used here include hydrolysis , dehydration , isomerization , aldol condensation , hydrogenation and oxidation . A frequent goal is on the one hand to reduce the functionality of the sugar in order to be able to use it better in industrial downstream processes, for example as polyol . Another goal is secondary products that have a lower degree of oxidation and thus a higher energy content than sugar. These products can optionally be used as fuels, but also as basic or intermediate products in the chemical industry. Attempts are also being made to develop chiral sugars for use as chiral pool components.
The structure of the most common or most important sugars as a renewable raw material, their origin and their use are explained below.
Simple sugars
In nature, sugars are rarely found as a monomer. This is why simple sugars are usually obtained from di- or polysaccharides.
glucose
The most common sugar is glucose (C 6 H 12 O 6 , also grape sugar or dextrose). The most important higher saccharides consist of glucose (the polysaccharides starch, cellulose) or contain glucose (the disaccharide sucrose from glucose and fructose). It can be obtained from starch, which is a polymer made from glucose.
- use
- Hydroxymethylfurfural can be cheaply made from glucose (or cellulose). The reaction is carried out in an ionic solvent with chromium (III) chloride as a catalyst . Yields of up to 70% of hydroxymethylfurfural are achieved under relatively mild reaction conditions. Hydroxymethylfurfural can be further processed to 2,5-dimethylfuran in a further step. This can also be used as fuel.
- Biotechnological products
- In most organisms, glucose is a central intermediate of metabolism from which many compounds can be derived. In biotechnological applications, glucose is therefore often used as a substrate for fermentations in which compounds such as ethanol ( bioethanol ), organic acids , amino acids , and antibiotics are produced. The most important application in terms of quantity is the production of ethanol, especially for use as a biofuel , with the help of yeast . The process is similar to the production of spirits in a distillery . In this case, the starting raw material is often starch (grain, corn, potatoes etc.), which is enzymatically broken down into glucose. The fermentative provision of raw materials for the production of plastics, such as polyhydroxyalkanoates or polylactides, is of increasing importance .
- For these applications, other sugars (sucrose, often in the form of molasses ) or other organic compounds (glycerine, etc.) can often be used instead of glucose .
Products made from various simple sugars
Some products made from simple sugars are based on chemical changes that are theoretically possible in analog form with all simple sugars. Mostly, however, glucose is used because of its good availability.
- use
- Sugar acids are used in many ways for industrial applications, such as in the food industry as an acid component, but also for technical applications. The most important representative is gluconic acid , which is obtained from glucose. The production usually takes place in a biochemical process, which is relatively uneconomical due to the necessary cleaning steps and the amount of by-products. More recent developments are based on the use of chemical catalysts.
- Through catalytic reduction, sugars can be converted into short-chain polyols. For example, ruthenium is used as a catalyst, and water is used as a solvent. The resulting polyols can be reacted with isocyanates to form polyurethanes and are used as paints.
- Sugars can also be used to make polymethyl methacrylate or acrylic glass. 2-Hydroxyisobutyrate is enzymatically produced from sugar. This is a precursor of the monomer methyl methacrylate, which can ultimately be polymerized to polymethyl methacrylate. The process is still in the development stage.
Double and multiple sugars
A disaccharide is obtained by glycosidic linkage of two identical or different monosaccharides.
Sucrose
The most common disaccharide is sucrose (table sugar). It consists of glucose and fructose. It is obtained from sugar cane or sugar beet.
- use
- The fermentation of sucrose from sugar cane to ethanol is of great importance for the domestic fuel supply in Brazil. In Europe, sucrose from sugar beet is also used to produce bioethanol.
Products made from various double and multiple sugars
Di- or oligosaccharides are required for some applications.
- use
- Alkyl polyglycosides are esters of fatty acids and sugar. The sugar (mono- to oligosaccharide) serves as the hydrophilic segment, the carbon chain of the fatty acid as the hydrophobic part of this non-ionic, biodegradable surfactant. Alkyl polyglycosides are now produced and used on an industrial scale.
Polysaccharides
Various polysaccharides occur frequently in nature and serve, for example, as energy stores (starch) or give plants their structure (cellulose in plant fibers or wood).
Strength
The starch is a polymer made from glucose. It consists of amylose , in which numerous glucose molecules are exclusively linked α-1,4-glycosidically. Another component is amylopectin , which is largely structured like amylose, but also contains a small number of α-1,6-glycosidic bonds to other amylose or amylopectin molecules. The amylase has a linear, spiral shape, the amylopectin is also branched. Therefore, the strength is not, such as B. cellulose, as a highly ordered structure and is therefore easily accessible to enzymes ( amylases ). Therefore, starch serves as an easily mobilized energy reserve in plants and is in the form of granules in the cells. Amylases are also used in biotechnological applications to break down starch into mono- and oligomers. B. first to make water soluble.
- use
- Bioethanol
- Starch from a wide variety of plants can be used as a raw material for the production of bioethanol . No starch isolation is necessary for this application, as pure alcohol can be obtained by distilling the fermented raw materials.
- Glucose : Starch is a homopolymer of glucose. A breakdown into this monomer is possible with amylases.
- Starch : If starch is required in its pure form, it can e.g. B. from grain and maize grain as well as potatoes (starch or potatoes) are obtained. It is required , among other things, in the manufacture of textiles, adhesives and paper ( paper starch ). Polymers can also be made from starch ( starch polymer ).
Cellulose
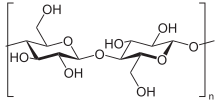
Cellulose is a polymer made from glucose. Due to the β-1,4-glycosidic linkage, the cellulose is linear. The parallel arrangement of numerous cellulose molecules creates fibrils with partially crystalline areas. This special structure can be found in plant fibers, for example, and gives them high tear resistance. Wood consists to a large extent of cellulose, which is additionally encrusted with lignin ( lignocellulose ).
- use
- Cellulose : An important material application is the production of cellulose , which mainly consists of cellulose. This is obtained by removing the lignin from wood and used as the main raw material in paper production.
- Cellulosic ethanol : For the production of cellulosic ethanol as a second-generation biofuel, plant parts are to be developed in the future that have not yet been fermented. Due to the ordered structure of the cellulose in plant fibers and the lignin components in wood, the breakdown into mono- or oligosaccharides has not been economically feasible up to now. The decomposition is necessary in order to be able to carry out the ethanol fermentation with yeast .
Sugar as a component of biomass
Sugar usually makes up the largest proportion of biomass. For example, plant fibers consist primarily of cellulose, and wood also consists of 70 to 80% of the polysaccharides cellulose and hemicellulose . In the material use of plant fibers and timber or in the energetic use of straw, wood, etc. through incineration, sugar is primarily used.
See also
literature
- Lubert Stryer: Biochemistry. 7th edition. Spectrum Academic Publishing House, Heidelberg / Berlin / Oxford et al. 2007, ISBN 978-3-8274-2988-9 .
Web links
- Uta Bilow: Crude oil was yesterday - sugar is tomorrow. at: faz.net , June 27, 2007.
Individual evidence
- ↑ Renewable raw materials: Sugar in the tank - spectrum-direct. www.wissenschaft-online.de, accessed on October 25, 2009 .
- ↑ Sugar - a promising renewable raw material. (No longer available online.) Www.profil.iva.de, formerly in the original ; Retrieved October 24, 2009 . ( Page no longer available , search in web archives )
- ↑ Alternative energies: New fuel from starch and cellulose - News Science - WELT ONLINE. www.welt.de, accessed on October 25, 2009 .
- ↑ Garabed Antranikian (Ed.): Applied Microbiology. Springer-Verlag, Berlin / Heidelberg 2006, ISBN 3-540-24083-7 .
- ↑ Archive - Publications - BMEL Research. In: bmel-forschung.de. Retrieved January 23, 2017 . FoRep 1/2009: Plants as renewable raw materials , pages 23-25
- ↑ Sugar as a raw material for polyurethane production. www.uni-protocol.de, accessed on October 25, 2009 .
- ↑ Acrylic glass made from sugar: Researchers are developing a new environmentally friendly method for producing acrylic glass. www.scinexx.de, accessed on October 24, 2009 .
- ^ Fats and oils as oleochemical raw materials, by Karlheinz Hill. (PDF; 60 kB) (No longer available online.) Surfactantspectator.com, formerly in the original ; Retrieved October 25, 2009 . ( Page no longer available , search in web archives )