Heptoses
Heptoses are monosaccharides with a carbon backbone containing seven carbon atoms. They all have the empirical formula C 7 H 14 O 7 and a molar mass of 210.18 g / mol. They differ in the type of carbonyl function. In the case of a keto group, one speaks of ketoheptoses , in the case of an aldehyde group it is called aldoheptoses . There are 32 stereoisomeric aldoheptoses and 16 stereoisomeric ketoheptoses.
Occurrence and use
Heptoses only play a subordinate role in nature. The few naturally occurring heptoses include:
- The D -Sedoheptulose , as sedoheptulose-7-phosphate as an intermediate of the pentose phosphate pathway occurs and much of the regeneration of the D -Ribulose in the Calvin cycle is involved. The 7-deoxy-sedoheptulose is as herbicide investigated.
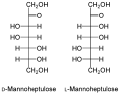
- The D -Manno-heptulose , in the avocado fruit occurs, and as hexokinase inhibitor is used. Oral or intravenous administration of D- mannoheptulose leads to a reduced release of insulin and thus prevents the blood sugar level from falling too quickly .
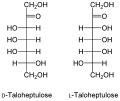
- The D -Talo-heptulose , which can be extracted from the avocado fruit.
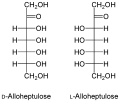
- The D- Allo-heptulose , which can be obtained from the avocado plant.
In addition, some heptoses are components of lipopolysaccharides (LPS).
Structure of the main ketoheptoses
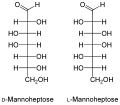
Structure of the main aldoheptoses
Physical Properties
| D -doheptulosis | D -Mannoheptulosis | D -Alloheptulosis | D -Taloheptulosis | D -Mannoheptosis | D -Glucoheptosis | |
| Molecular formula | C 7 H 14 O 7 | C 7 H 14 O 7 | C 7 H 14 O 7 | C 7 H 14 O 7 | C 7 H 14 O 7 | C 7 H 14 O 7 |
| CAS number | 3019-74-7 | 3615-44-9 | 7101-28-2 | 31297-62-8 | 7634-39-1 | 62475-58-5 |
| PubChem | 102926 | 12600 | ||||
| M.p. (° C) | 100-102 | 151-152 | 130-132 | 135-137 | 145 | 180 |
| Appearance | White dust | White dust | White dust | White dust | White dust | White dust |
See also
Individual evidence
- ↑ Stryer L. Biochemistry . 4th edition. Spektrum Akademischer Verlag, 2004, p. 447.
- ↑ E. Simon, G. Frenkel, PF Kraicer: Blockade of insulin secretion by mannoheptulose. In: Israel Journal of Medical Sciences. Vol. 8, No. 6, 1972, ISSN 0021-2180 , pp. 743-752.
- ↑ a b Ingvar Johansson, Nelson K. Richtmyer: The isolation of Both a talo-heptulose and to allo-heptulose from the avocado. In: Carbohydrate Research . Vol. 13, 1970, pp. 461-464, doi: 10.1016 / S0008-6215 (00) 80607-2 .
- ↑ a b Peter M. Collins (Ed.): Dictionary of Carbohydrates. With CD-ROM. 2nd Edition. Chapman & Hall / CRC Press, Boca Raton FL et al. 2006, ISBN 0-8493-3829-8 , p. 607.
- ↑ a b Peter M. Collins (Ed.): Dictionary of Carbohydrates. With CD-ROM. 2nd Edition. Chapman & Hall / CRC Press, Boca Raton FL et al. 2006, ISBN 0-8493-3829-8 , p. 609.
- ^ Joseph H. Roe, CS Hudson: Further Studies of the Physiological Availability of Heptoses. (PDF; 290 kB) In: The Journal of Biological Chemistry. Vol. 121, No. 1, 1937. pp. 37-43.
- ↑ Dennis R. Heldman, Daryl B. Lund (Ed.): Handbook of Food Engineering (= Food Science and Technology. Vol. 161). 2nd Edition. CRC Press, Boca Raton FL et al. 2006, ISBN 0-8247-5331-3 , p. 339 .