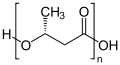
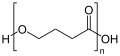
Polyhydroxyalkanoates
Polyhydroxyalkanoates (PHA) or polyhydroxy fatty acids (PHF) are naturally occurring, water-insoluble and linear biopolyesters that are formed by many bacteria as reserves for carbon and energy . These biopolymers are biodegradable and are used to manufacture bio-based plastics .
structure
The simplest and most common form of PHA is poly [( R ) -3-hydroxybutyrate] ( polyhydroxybutyric acid , PHB or poly (3HB)) synthesized by fermentation . This consists of 1,000 to 30,000 hydroxy fatty acid units . In addition to 3-hydroxybutyric acid , around 150 other hydroxy fatty acids are known as PHA building blocks.
PHA can either be short-chain (short-chain length PHA, scl-PHA) with 3 to 5 carbon atoms, medium-chain length PHA, mcl-PHA) with 6 to 14 carbon atoms or long-chain length PHA, lcl – PHA) with 15 or more carbon atoms can be synthesized. Depending on the microorganism and cultivation conditions, homo- or copolyesters are produced with a wide variety of hydroxycarboxylic acids .
PHA types
PHA monomers: polyhydroxybutyrate, polyhydroxyvalerate PHA co-polymers: P (4hb-Co-3hb), P (3hb-Co-3hv) PHA terpolymers: P (3hb-Co-3hv-Co-4hb).
PHAs are structured according to the following structural formula:
| Alkyl branch | Surname | abbreviation |
|---|---|---|
| R = H | Poly (3-hydroxypropionate) | (PHP) |
| R = CH 3 | Poly (3-hydroxybutyrate) | (PHB, P3HB) |
| R = CH 2 CH 3 | Poly (3-hydroxyvalerate) | (PHV) |
| R = propyl | Poly (3-hydroxyhexanoate) | (PHHx) |
| R = butyl | Poly (3-hydroxyheptanoate) | (PHH) |
| R = pentyl | Poly (3-hydroxy octanoate) | (PHO) |
| R = hexyl | Poly (3-hydroxynonanoate) | (PHN) |
| R = heptyl | Poly (3-hydroxydecanoate) | (PHD) |
| R = octyl | Poly (3-hydroxy undecanoate) | (PHUD) |
| R = nonyl | Poly (3-hydroxy dodecanoate) | (PHDD) |
| R = undecyl | Poly (3-hydroxytetradecanoate) | (PHTD) |
| R = dodecyl | Poly (3-hydroxypentadecanoate) | (PHPD) |
| R = tridecyl | Poly (3-hydroxyhexadecanoate) | (PHHxD) |
A "-Co-" is used to indicate the copolymer
| Co-polymer name | abbreviation | |
|---|---|---|
| poly (3-hydroxypropionate-co-3-hydroxybutyrate) | (P3HP-3HB) | |
| poly (3-hydroxypropionate-co-4-hydroxybutyrate) | (P3HP-4HB) | |
| poly (3-hydroxybutyrate-co-4-hydroxybutyrate) | (P (3HB-4HB)) | |
| poly (3-hydroxybutyrate-co-3-hydroxyvalerate) | (PHBV) | |
| poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) | (PHBV-HHx) | |
| R = C3-C11 | medium chain length PHA | (mcl-PHA) |
| R = C12 and more | long chain length PHA | (lcl-PHA) |
biosynthesis
PHAs are synthesized in bacterial cells through a metabolic process . The substrates for the biosynthetic PHAs are usually limited to small molecules , since bacteria have thick, rigid cell walls as membranes . Large polymeric molecules cannot be transported into the cell, and extracellular transformation either by the microorganism or by a chemical process is necessary for the polymeric molecules to be used.
In order to make the glucose building blocks of the sucrose substrates available for microbial PHA production, the substrates are often hydrolyzed beforehand .
The biosynthesis of PHA by microorganisms is usually triggered during fermentation by certain deficiency conditions (e.g. deficiency in the macro-elements phosphorus , nitrogen , deficiency in trace elements or oxygen deficiency) with a simultaneous oversupply of carbon sources. JoAnne Stubbe and her group researched and isolated a first enzyme for biosynthetic PHA synthase and investigated the polymers built up by the enzyme. A list of enzymes involved in the PHA biosynthetic pathway is given in Tan et al. compiled.
The biopolyesters are deposited in the cells in the form of water-insoluble, highly refractive granules as energy storage materials . Most PHA-synthesizing microorganisms can use simple sugars as substrates . The hydrocarbon metabolism of triacylglycerol ( fats and oils ) is more limited, but can be carried out by species of Pseudomonas bacteria. Different bacteria can produce PHAs with a different composition from the same substrate.
Homopolyesters are formed with pure substrates. If co-substrates such as valeric acid or glycerine are added to the main substrates , the microorganisms produce co-polyesters with different hydroxycarboxylic acids.
Here are some selected strains of microorganisms that synthesize a high concentration of PHA in dry cell mass from substrates:
| Carbon source group | Carbon source | Microorganism strain | PHA |
|---|---|---|---|
| Hydroxycarboxylic acids |
3-hydroxybutanoic acid , 4-hydroxybutanoic acid |
Eutropha N9A and Wautersia eutropha | P3HB |
| Hydroxycarboxylic acids | Alkenes , n - alkanes |
Pseudomonas putida GPo1, Pseudomonas oleovorans |
scl-mcl-PHA, mcl-PHA |
| Glycerin | Glycerin | Burkholderia cepacia | P3HB |
| Glycerin | Raw glycerine from biodiesel production | Haloferax mediterranei | P3HB3HV |
| Polysaccharides | glucose | CECT 4623, KCTC 2649, NCIMB 11599, Novosphingobium nitrogenifigens Y88, Ralstonia eutropha |
P3HB |
| Polysaccharides | Fructose + glucose |
Azohydromonas lata , Alcaligenes latus, Cupriavidus necator H16 (formerly Hydrogenomonas eutropha H16) (formerly Alcaligenes eutrophus H16) (former Ralstonia eutropha H16) (former Wautersia eutropha H16), Burkholderia cepacia, Pseudomonas multivorans, Pseudomonas cepacia |
P3HB |
| Polysaccharides | Glucose + valeric acid | Caldimonas taiwanensis | PHBV (49% B-51% V) |
| Polysaccharides | Glucose + lauric acid |
Aeromonas hydrophila, Aeromonas caviae, Rhodospirillium rubrum, Rhodocyclus gelatinosus , Sinorhizobium fredil |
P (3HB-co-3HHx), P (3HB-co-3HO), SCL-MCL copolymers |
| Polysaccharides | Glucose + medium-chain fatty acids | Aeromonas hydrophila, Cupriavidus necator, Hydrogenomonas eutropha, (formerly Alcaligenes eutrophus), (formerly Ralstonia eutropha) and (formerly Wautersia eutropha), Caldimonas taiwanensis |
P3HB-co-3HHx, P3HB-co-3HV |
| Polysaccharides | Sucrose | Azohydromonas lata, (formerly Alcaligenes latus), |
P3HB |
| Polysaccharides | Xylans | Co-culture of Saccharophagus degradans and Bacillus cerues, Burkholderia cepacia, Pseudomonas multivorans and Pseudomonas cepacia, P. cepacia |
P3HB |
| Sugar molasses | Sugar beet molasses | Haloferax mediterranei DSM 1411 | PHBV (86-14) |
| Sugar molasses | Beet molasses | Alicaligenes latus, Ralstonia eutropha, Haloferax meduterranel, Azotobacter vinelundi |
P3HB, P (3HB-3HV), P (3HB-4HB) |
| Sugar molasses |
Sugar cane molasses + fructose, glucose, sucrose, glycerin |
Pseudomonas aeruginosa NCIM 2948 | P3HB |
| Sugar molasses | Malt- sugar waste |
Azohydromonas australica, (formerly Alcaligenes latus), Azotobacter vinelandii |
P3HB |
| Polysaccharide starch | Hydrolyzed potato starch | Halomonas boliviensis LC1 | P3HB |
| Polysaccharide starch | Hydrolyzed Potato Starch + Valeric Acid , Hydrolyzed Wheat Starch + Valeric Acid |
Caldimonas taiwanensis | PHBV (80-10) |
| Polysaccharide starch | Hydrolysed tapioca (cassava) + valeric acid, corn starch + valeric acid |
Caldimonas taiwanensis, |
PHBV (87-13) |
| fats and oils | Vegetable oils | Ralstonia eutropha | P3HB |
| fats and oils |
Olive oil , corn oil , palm oil , oleic acid |
Cupriavidus necator H16, (formerly Hydrogenomonas eutropha H16) , (formerly Alcaligenes eutrophus H16), (formerly Ralstonia eutropha H16), Wautersia eutropha H16 |
P3HB |
| fats and oils | olive oil | Aeromonas hydrophilia, Aeromonas caviae |
mcl-PHA, P3HB-3HHX |
| fats and oils | Waste water from the olive oil mills | Haloferax mediterranei DSM 1411 | PHBV (94-6) |
| fats and oils |
Palm kernel oil , raw palm oil, fatty acids from palm oil and palm kernel oil |
Cupriavidus necator | mcl-PHA |
| fats and oils | Soybean oil | Pseudomonas stutzeri | mcl-PHA |
| fats and oils |
Peanut oil , castor oil , mustard oil , sesame oil |
Comamonas testosteroni | P3HB |
| fats and oils | Mustard oil | Pseudomonas aeruginosa | PHA |
| fats and oils |
Coconut oil , sebum oil |
Pseudomonas saccharophilia | mcl-PHA |
| fats and oils | Tallow- based biodiesel | Pseudomonas citronellolis, Pseudomonas oleovorans, Pseudomonas stutzeri |
mcl-PHA, P3HHX, P3HO |
| fats and oils | Cooking oil waste | Ralstonia eutropha | P3HB, P (3HB-3HV) |
| Lactose , milk sugar | whey | Escherichia coli harboring A. latus genes | P3HB |
| Lactose, milk sugar | hydrolyzed whey | Haloferax mediterranei | P3HB3HV |
| Lactose, milk sugar | Lactose + sucrose | Hydrogenophaga pseudoflava ATCC 33668, DSM 1034 |
P3HB3HV |
| Alcohols | Methanol | Methylobacterium extorquens, Methylobacterium. organophilum |
P3HB |
| Fatty acids |
Lauric acid , myristic acid , palmitic acid , stearic acid , oleic acid |
Burkholderia sp. USM JCM 15050 | P3HB |
| Fatty acids | Pelargonic acid | Pseudomonas putida KT2440 | mcl-PHA |
| Fatty acids in algae | Agarose | Co-culture of Saccharophagus degradans and Bacillus cerues | P3HB |
| Cellulose | Hemicellulose hydrolyzate | Burkholderi cepacia ATCC 17759 | P3HB |
| Cellulose | Cellulose Sigmacell | Saccharophagus degradans | P3HB |
| Cellulose | α-cellulose | Saccharophagus degradans | P3HB |
| Cellulose | silage | Haloferax mediterranei DSM 1411 | PHBV (85-15)
|
| gaseous hydrocarbons | methane | Methylotroph spp. yeast | P3HB |
| gaseous hydrocarbons | Carbon dioxide , CO 2 | Cupriavidus necator H16, (formerly Hydrogenomonas eutropha H16), (formerly Alcaligenes eutrophus H16), (formerly Ralstonia eutropha H16), (formerly Wautersia eutropha H16) |
P3HB |
| liquid hydrocarbons | n- octane | Pseudomonas oleovorans, Pseudomonas citronellolis |
mcl-PHA, P3HHx, P3HO, P3HD |
| liquid hydrocarbons |
Benzene , ethylbenzene , toluene , styrene , p -xylene |
Pseudomonas fluva TY16, Pseudomonas putida F1, Pseudomonas putida CA-3 |
mcl-PHA |
| Nucleic bases , nucleic acids |
Adenine , purines |
Blastobotrys adeninivorans | PHA |
| nutrient | Nutrients in salt lakes with a high salt concentration | Methyl aspartate cycle | PHA |
The literature works cited contain extensive tables with: microorganism strains, carbon sources, type of PHAs formed, dry matter of bacteria, PHA in the fermentation solution, proportion of PHA in dry cell mass and yield of PHA based on the amount of substrate. The complete tables in the articles can be downloaded as full text from the DOI.
properties
Depending on the chemical composition (homo- or copolyester, hydroxycarboxylic acids contained), the properties of the PHAs differ :
- Non-toxic, therefore suitable for food packaging .
- Biocompatible and therefore suitable for medical applications.
- Good resistance to moisture .
- Show a low permeation of water .
- Have aroma barrier properties.
- Good resistance to ultraviolet radiation .
- Poor chemical resistance to acids and bases .
- In water insoluble.
- Soluble in chloroform and other chlorinated hydrocarbons such as dichloromethane .
- Relatively resistant to hydrolytic degradation .
- Heavier than water, which facilitates anaerobic biodegradation in sediments .
- The crystalline proportion can range from a few to 70%.
- Less sticky than traditional melted polymers.
- Tolerated in contrast to other bio-based plastics such as polymers of polylactic acid partial temperatures up to 180 ° C.
- PHB polymer synthesized purely from polyhydroxybutyric acid is relatively brittle and stiff .
- Processability , impact resistance and elasticity are improved by a higher proportion of valeriate in the material.
- PHB copolymers that also contain other fatty acids such as B. contain beta-hydroxyvaleric acid, increase elasticity .
| property | abbreviation | [Unit] | Homopolymer scl-PHA | Homopolymer mcl-PHA | Copolymer P (3HB-co-3HV) | Copolymer P (3HB 94-co-3HD 6) |
|---|---|---|---|---|---|---|
| Melting temperature | Tm | [° C] | 160-179 | 80 | 137-170 | 130 |
| Glass transition temperature | Day | [° C] | 2-4 | −40 | −6 to 10 | −8 |
| Degree of crystallization | Xcr | [%] | 40-60 | |||
| Young's modulus , Young's modulus | E-modul | [GPa] | 1-3.5 | 0.7-2.9 | ||
| tensile strenght | [MPa] | 5-15 | 20th | to 690 | 17th | |
| Elongation at break | ε | [%] | 1-40 | 300 | 30-38 | 680 |
| Water vapor transmission rate | WVTR | [g · mm / m² · day] | 2.36 | |||
| Oxygen transmission rate | OTR | [cc · mm / m² · day] | 55.12 |
PHA extraction
The extraction of PHA from biomass uses a sequence of different techniques:
- Biomass harvest
Biomass harvest is the concentration of biomass using techniques such as filtration or centrifugation .
- Pretreatment and destruction of cell membranes
Since PHAs are intracellular polymers, it is necessary to concentrate the biomass prior to PHA recovery. Techniques include drying techniques ( lyophilization and thermal drying), grinding, chemical, enzymatic and biochemical pre-treatments. The pre-treatment step can combine two or more methods.
- PHA accumulation
The destruction of the non-PHA cell mass (NPCM) can be mechanical, chemical, enzymatic, biological or osmotic .
- PHA extraction
In PHA solubilization , the PHA is precipitated through the use of an alcohol. The solvents used are “aqueous, glycol-containing two-phase systems”, “ halogenated solvents ”, “non-halogenated solvents” or “ extraction with supercritical liquids ”.
- Polishing and drying.
As a final step, the recovered PHAs can be polished by removing residues from the previous steps or drying them.
Industrial manufacturing
The main PHA producers are: Bioamber, Inc .; Bioscience; Biomatera, Inc .; Biome Bioplastics Ltd.; Biomer; Bio-On-Srl; Biotechnology Co., Ltd; Bluepha Co., Ltd .; Cardia Bioplastics Ltd; CJ Cheiljedang Corp .; Dayglo Color Corp .; Danimer Scientific; Dupont Tate & Lyle Bio Products Company, LLC; Full cycle bioplastics; Kaneka Corporation; Meredian Holdings Group, Inc .; Metabolix Inc .; Newlight Technologies, LLC; PHB Industrial SA; Polyferm Canada, Inc .; Procter & Gamble Co .; Shenzhen Ecomann Biotechnology Co., Ltd .; Tepha, Inc .; Tianan Biologic Materials Co. Ltd .; Tianjin Greenbio Materials Co. Ltd.
A list of production sites and capacities is given in polyhydroxybutyric acid .
Processing and use
PHA polymers have great potential as a substitute for bulk plastics such as e.g. B. Polypropylene (PP), especially in the field of packaging and coatings . PHA's share of 2017 global bioplastics capacities of 2.05 million / year is 2.4%.
PHA polymers are thermoplastic on conventional equipment to process and are deformed depending on the composition and more or less elastic.
PHA are mainly processed in injection molding , by extrusion and extrusion blow molding into films and hollow bodies . PHA is a thermoplastic that can be used as a melt material in 3D printing . The product shapes can be reshaped and designed by bending, pressure, tension and tension . This is how bottles, golf tees, pens and containers for cosmetics are created.
Plastics made from PHA are used as biodegradable elastomers and thermoplastics , for example for packaging material, especially for food . Straws made from it are resistant to hot liquids without changing the taste of the drinks.
They are also used in the medical field, e.g. B. as absorbable by the body materials such as sutures , for implants and for pharmaceutical depot preparations . Their use as hygiene articles (e.g. diaper components ), fibers , adhesives, components of toner and developer fluids , carriers of flavorings in food and biodegradable fishing nets is tried and tested.
In agriculture, PHAs can e.g. B. as films or as mulch and in aquaculture as a biofilm carrier for denitrification .
Depending on the co-monomer composition and molecular weight, the PHAs can be used as: multifilament ; Spunbond ; Synthetic paper; as latex for paper coating, foams ; Injection molding ; Rigid blown forms ; Thermoforming ; Blown films and sheets; Film for molds ; Elastomeric film; Adhesion promoter ; Glue .
PHA as a thickener and binder in technical lubricants.
ecology
PHA disintegrate relatively quickly and 100% during biodegradation in industrial composting and in biogas plants as well as on the domestic compost heap, in soil and in the sea. The biological decomposition process can take place in the air as well as in water. So to build implants such as screws for bone fractures are used, or surgical sutures from without further intervention. However, drugs and active ingredients incorporated into PHA can also be used for a targeted release in the human body.
Their use is described in biodegradable solvents and as electrically conductive polymers.
Web links
- Biopolymers - raw materials for innovative medical products on biooekonomie-bw.de, accessed on November 8, 2018.
- Zulfiqar Ali Raza, Sharjeel Abida, Ibrahim M.Banat: Polyhydroxyalkanoates: Characteristics, production, recent developments and applications, Review . (pdf) In: International Biodeterioration & Biodegradation . 126, January 2018, pp. 45-56. doi : 10.1016 / j.ibiod.2017.10.001 .
- Harvey Williams, Patricia Kelly: Polyhydroxyalkanotes; Biosynthesis, Chemical Structures and Applications , Nova, Complimentary Contributor Copy
- Elodie Bugnicourt; Patrizia Cinelli; Vera Alejandra Alvarez; Andrea Lazzeri: Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging, eXPRESS Polymer Letters 8 (11): 791-808 June 2014
- Global Polyhydroxyalkanoate (PHA) Market Forecast, Marketing Channels, Major Industry Participants, and Strategies To 2024, accessed July 26, 2019
Individual evidence
- ^ A b Elizabeth C. Wittenborn, Marco Jost, Yifeng Wei, JoAnne Stubbe, Catherine L. Drennan ,: Structure of the Catalytic Domain of the Class I Polyhydroxybutyrate Synthase from Cupriavidus necator . (pdf) In: Journal of Biological Chemistry . 291, No. 48, October 2016, pp. 25264-25277. doi : 10.1074 / jbc.M116.756833 . PMID 27742839 . PMC 5122792 (free full text).
- ↑ a b Giin-Yu Amy Tan, Chia-Lung Chen, Ling Li, Liya Ge, Lin Wang, Indah Mutiara Ningtyas Razaad, Yanhong Li, Lei Zhao, Yu Mo and Jing-Yuan Wang: Start a Research on Biopolymer Polyhydroxyalkanoate (PHA ): A Review ' . (pdf) In: Polymers . 6, No. 3, March 2014, pp. 706-754. doi : 10.3390 / polym6030706 .
- ↑ a b Jia Yu: Ralstonia eutropha in Microbial Production of Bioplastics from Renewable Resources 2007
- ↑ a b Nyok Lau Lau, Kumar Sudesh: Revelation of the ability of Burkholderia sp. USM (JCM 15050) PHA synthase to polymerize 4-hydroxybutyrate monomer . In: AMB Express . 2, No. 1, August 2012, p. 41. doi : 10.1186 / 2191-0855-2-41 . PMID 22877240 . PMC 3434029 (free full text).
- ↑ a b A.J. Anderson, Dawes, First2 = EA: Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. . In: Microbiology Reviews . 54 pages = 450-472, April 1990. PMID 2087222 .
- ↑ Guozhan Jiang, David J. Hill, Marek Kowalczuk, Brian Johnston, Grazyna Adamus, Victor Irorere, Iza Radecka: Carbon Sources for Polyhydroxyalkanoates and an Integrated Biorefinery . In: International Journal of Molecular Science . 17, No. 7, July 2016, p. 1157. doi : 10.3390 / ijms17071157 . PMID PMID 27447619 . PMC 4964529 (free full text).
- ↑ a b c d Elodie Bugnicourt, Patrizia Cinelli, Vera Alvarez, Undrea Lazzeri: Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging . In: eXPRESS Polymer Letters . 8, No. 11, 2014, pp. 791-808. doi : 10.3144 / expresspolymlett.2014.82 .
- ↑ a b Justyna Mozejko-Ciesielska, Robert Kiewisz: Bacterial polyhydroxyalkanoate: Still fabulous? . In: Microbiological Research . 192, March 2016, pp. 271-282. doi : 10.1016 / j.micres.2016.07.010 .
- ↑ Martin Koller: Advances in Polyhydroxyalkanoate (PHA) Production . In: Bioengineering (Basel). . 4, No. 4, December 2017, p. 88. doi : 10.3390 / bioengineering4040088 . PMID 29099065 . PMC 5746755 (free full text).
- ↑ a b Constantina Kourmentza, J. Plácido, N. Venetsaneas, A. Burniol-Figols, C. Varrone, HN Gavala, MA Reis: Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production (Review) . In: Bioengineering . 4, No. 2, June 2017, pp. 8-50. doi : 10.3390 / bioengineering4020055 . PMID 28952534 . PMC 5590474 (free full text).
- ↑ a b Shankar Aditi, D'Souza Shalet, Narvekar Manish, Rao Pranesh, Tembadmani Katyayini: Microbial production of polyhydroxyalkanoates (PHA) from novel sources: A Review . (pdf) In: International Journal of Research in Biosciences . 4, No. 4, October 2015, pp. 16-28. ISSN 2319-2844.
- ↑ Emilia Inone-Kauffmann: Polyhydroxy fatty acids (PHF). In: Hans Domininghaus: The plastics and their properties. 6th edition, Springer Verlag, 1990, ISBN 3-540-21410-0 , pp. 1451-1454.
- ↑ Nicolas. Jacquel, Chi ‐ Wei Lo, Ho ‐ Shing Wu, Yu ‐ Hong Wei, Shaw S. Wang: Solubility of polyhydroxyalkanoates by experiment and thermodynamic correlations . In: AIChE J . 53, No. 10, 2007, pp. 2704-2714. doi : 10.1002 / aic.11274 .
- ↑ Martin Koller, Horst Niebelschütz, Gerhart Braunegg: Strategies for recovery and purification of poly [(R) ‐3 ‐ hydroxyalkanoates] (PHA) biopolyesters from surrounding biomass . In: Engineering in Life Science . 13, No. 6, November 2013, pp. 549-556. doi : 10.1002 / elsc.201300021 .
- ↑ Mohamed H. Madkour ‡, Daniel Heinrich †, Mansour A. Alghamdi ‡, Ibraheem I. Shabbaj ‡, and Alexander Steinbüchel: PHA recovery from biomass . In: Biomacromolecules . 9, No. 14 (9), September 2013, pp. 2963-2972. doi : 10.1021 / bm4010244 . PMID 23875914 .
- ↑ Bioplastic market data accessed November 8, 2018.
- ↑ Researchers create the first straws using polyhydroxyalkanoate (PHA) plastic
- ↑ a b Polyhydroxyalkonate material properties accessed on November 8, 2018.
- ↑ K. Shantini; Kai-Hee Huong; Hema Ramachandran; AA Amirul: Microbial Production of Polyhydroxyalkanoates for Agricultural and Aquacultural Applications , Beneficial Microorganisms in Agriculture, Aquaculture and Other Areas pp 129-164
- ↑ Great progress in the development of environmentally friendly lubricants