Chlorinated hydrocarbons
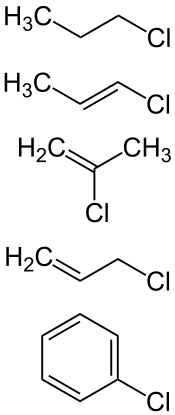
chloroalkane (1-chloropropane), chloroalkenes (1-chloropropene, 2-chloropropene, 3-chloropropene),
chloroaromatic (chlorobenzene)
Chlorinated hydrocarbons form a group of organic compounds and a subgroup of halogenated hydrocarbons . These chemical substances have a hydrocarbon skeleton in which one or more hydrogen atoms are replaced by chlorine . Their chemical properties make them almost indispensable for industry, on the other hand they usually have a great potential to damage the environment.
Many crop protection products , especially herbicides and insecticides , contain organochlorine compounds. They also play an important role in the manufacture of plastics ( e.g. PVC ) or as flame retardants . Numerous substances such as PCB and lindane , which have long been considered extremely beneficial and widely used, have now been banned again because of their proven harmfulness to humans and the environment. Such compounds played a central role in some environmental scandals in recent decades, such as the PCB pollution of the Krupa .
Classification and characteristics
The many known chlorinated hydrocarbons can be divided into the aliphatics chloroalkanes and chloroalkenes as well as aromatic chlorinated hydrocarbons. With increasing degree of chlorination, the stability and lipophilicity (fat solubility) of the substances increase. This makes absorption more difficult for degrading microorganisms and the activation energy required to degrade the substances increases. The increased hydrophobicity (water insolubility) of the substances also causes an accumulation in animal fat tissue.
Due to the high electronegativity of chlorine, many chlorinated hydrocarbons have very pronounced dipole moments , which means that they often result in high dielectric constants .
- Chloroalkanes
- Methyl chloride
- Dichloromethane
- Trichloromethane (common name chloroform ),
- Tetrachloromethane ( carbon tetrachloride ),
- Lindane ( γ-hexachlorocyclohexane ) is a cyclic representative of the chloroalkanes.
- Chloroalkenes
- Chloroalkynes
- Aromatic chlorinated hydrocarbons
- Chlorobenzene ,
- 1,4-dichlorobenzene ,
- DDT ( dichlorodiphenyltrichloroethane ).
- Oxygen- or nitrogen-containing derivatives of chlorinated hydrocarbons
- CS gas ( 2-chlorobenzylidenemalonic acid dinitrile ),
- TCDD (the most toxic agents from the class of dioxins ).
- Carboxylic acid chlorides - e.g. B. acetyl chloride , a derivative of acetic acid
Strictly speaking, these are not chlorinated hydrocarbons, as they contain other elements such as nitrogen and oxygen in addition to carbon, hydrogen and chlorine.
history
Organochlorides have been produced synthetically since the 1930s, for example by photochlorination . The introduction of a chlorine atom into the carbon structure often results in a reduction in the flammability of an organic compound. Organochlorines are therefore often used as non-flammable organic solvents , hydraulic oils and refrigerants . They are also used as synthesis precursors or crop protection agents.
Natural occurrence
For a long time it was assumed that there were hardly any natural sources of chlorinated organic compounds. However , in the last few years, high-performance analytics have been able to detect more and more natural organohalogens such as bipyrrole Q1 .
Wood-degrading fungi are an important source of naturally formed, chlorinated aromatic compounds . Interestingly, some fungi are even capable of de novo synthesis of chloroanisyls from glucose .
There are now over 3,800 known organohalogens of natural origin. With the exception of methyl chloride produced in the oceans and bipyrrole Q1 , the concentrations of most other compounds are far below those of anthropogenic origin. The great diversity of naturally occurring halogenated organic compounds and their widespread use is certainly one of the reasons for the degradability of anthropogenic pollutants.
See also
literature
- Otto Hutzinger : God created 91 elements, man a little more than a dozen and the devil one - chlorine . In: Environmental Sciences and Pollutant Research , 1990, 2, 2, p. 61, doi : 10.1007 / BF02936893 .
Individual evidence
- ↑ E. de Jong, AE Cazemier, JA Field and JAM De Bont (1994). Physiological role of chlorinated aryl alcohols biosynthesized de novo by the white rot fungus Bjerkandera sp. strain BOS55 . Appl Environ Microbiol 60 (1): pp. 271-277.
- ↑ E. de Jong, JA Field, H.-E. Spinnler, JBPA Wijnberg and JAM De Bont (1994). Significant biogenesis of chlorinated aromatics by fungi in natural environments . Appl Environ Microbiol 60 (1): pp. 264-270.
- ^ EJ Hoekstra, EWB De Leer (1995). Organohalogens: The natural alternatives . Chem Br February: pp. 127-131.
- ^ Gordon W. Gribble: The diversity of naturally produced organohalogens. In: Chemosphere . 52, 2003, pp. 289-297, doi : 10.1016 / S0045-6535 (03) 00207-8 .
- ↑ MM Häggblom, VK Knight, LJ Kerkhof (2000). Anaerobic decomposition of halogenated aromatic compounds . Environmental Pollution 107: pp. 199-207.