Dichloroethine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dichloroethine | |||||||||||||||
| other names |
Dichloroacetylene |
|||||||||||||||
| Molecular formula | C 2 Cl 2 | |||||||||||||||
| Brief description |
colorless liquid with an unpleasant, sweet odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 94.93 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.952 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−64 ° C |
|||||||||||||||
| boiling point |
32 ° C |
|||||||||||||||
| solubility |
soluble in ethanol , diethyl ether , acetone |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 0.1 ml m −3 or 0.4 mg m −3 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Dichloroethine is an organic chlorocarbon compound that can be viewed as a chlorinated derivative of ethyne .
history
The compound was first mentioned as an intermediate in 1918 when a tolane was synthesized with the starting materials calcium carbide , chlorine and benzene . The first production was described in 1930 from trichloroethene in the presence of potassium hydroxide and calcium oxide at 130 ° C and from ethyne and chlorine.
Extraction and presentation
A large number of versions of dichloroethine are based on the action of alkali hydroxides or alkali lye on trichloroethene. The dehydrohalogenation of trichloroethene can also be achieved using lithium bis (trimethylsilyl) amide at −78 ° C. This synthesis in the presence of diethyl ether gives a more stable 1: 1 adduct. Production with high yield can be carried out by reacting trichlorethylene with potassium hydride in the presence of a catalytic amount of methanol in tetrahydrofuran . The direct chlorination of ethyne is achieved using potassium hypochlorite .
Pyrolysis of dichloromaleic anhydride at 850 ° C also gives the compound.
properties
Dichloroethine is a very volatile liquid that boils at 32 ° C under normal pressure . The heat of vaporization is 27.4 kJ · mol −1. With a heat of formation of 199 kJ · mol −1 , it is a strongly endothermic compound that tends to decompose explosively and ignites spontaneously in air. A stable 1: 1 compound with diethyl ether containing 55.4% dichloroethine is not explosive and stable in air.
use
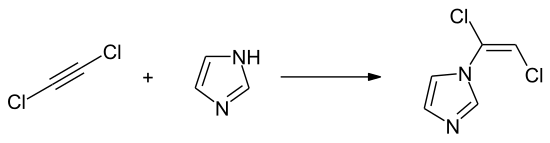
The compound is not used commercially because of its highly harmful effects and chemical instability. In organic synthesis, the 1: 1 adduct with diethyl ether can be used to introduce a vinyl, ethynyl or dichlorovinyl function into organic molecules. The implementation z. B. with imidazole gives the N - (1,2-dichlorovinyl) imidazole.
toxicology
According to the Technical Rules for Hazardous Substances (TRGS) 905, the substance is classified as carcinogenic for humans in category K1B.
Individual evidence
- ↑ a b c d e f Entry on dichloroacetylene in the GESTIS substance database of the IFA , accessed on February 8, 2020(JavaScript required) .
- ↑ a b c John Wotiz, Francis Huba, Robert Vendley: Notes α-Chloroacetylenes. In: The Journal of Organic Chemistry. 26, No. 5, 1961, pp. 1626-1627, doi: 10.1021 / jo01064a600 .
- ↑ a b IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans: Dichloroacetylene , 39 (1986), 369-78.
- ↑ Entry on dichloroacetylene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 7572-29-4 or dichloroethine ), accessed on November 2, 2015.
- ↑ Clinton Davidson: Tolane chlorides from calcium carbide, chlorine and benzene. In: Journal of the American Chemical Society. 40, No. 2, 1918, pp. 397-400, doi: 10.1021 / ja02235a009 .
- ↑ Erwin Ott, Walter Ottemeyer, Kurt packing Dorff: About acetylene dichloro-that. In: Reports of the German Chemical Society (A and B Series). 63, No. 8, 1930, pp. 1941-1944, doi: 10.1002 / cber.19300630810 .
- ↑ Patent DE495787 : Process for replacing hydrogen on triple bonded carbon with chlorine and bromine. Published on April 17, 1930 , applicant: IG Farben AG.
- ↑ Entry on trichlorethylene. In: Römpp Online . Georg Thieme Verlag, accessed on November 27, 2014.
- ↑ a b c Kende, AS; Fludzinski, P .: A Convenient Laboratory Synthesis of Dichloroacetylene. In: Synthesis . 1982, 455-456. doi: 10.1055 / s-1982-29831 .
- ↑ Jean Noel Denis, Albert Moyano, Andrew E. Greene: Practical synthesis of dichloroacetylene. In: The Journal of Organic Chemistry. 52, No. 15, 1987, pp. 3461-3462, doi: 10.1021 / jo00391a059 .
- ↑ Fritz Straus, Leo Kollek, Walther Heyn: About the replacement of positive hydrogen by halogen. In: Reports of the German Chemical Society (A and B Series). 63, 1930, pp. 1868-1885, doi: 10.1002 / cber.19300630739 .
- ↑ RM Trifu: Homopolymers of Dihaloacetylenes . Dissertation, University of Illinois at Chicago 1999, ISBN 978-0-549-39503-4 ( limited preview in Google Book Search).
- ↑ a b Jeffrey A. Manion: Evaluated Enthalpies of Formation of the Stable Closed Shell C1 and C2 Chlorinated Hydrocarbons. In: Journal of Physical and Chemical Reference Data . 31, No. 1, 2002, pp. 123-172, doi: 10.1063 / 1.1420703 .
- ↑ a b P.G. Urben; MJ Pitt: Bretherick's Handbook of Reactive Chemical Hazards . 6th Edition, Vol. 1, Butterworth / Heinemann 1999, ISBN 0-7506-3605-X , p. 229.
- ↑ Erwin Ott: About dichloroacetylene, III. Mitteil .: Presentation and some lecture attempts with the safe to handle molecular compound with ether. In: Reports of the German Chemical Society (A and B Series). 75, No. 12, 1942, pp. 1517-1522, doi: 10.1002 / cber.19420751215 .