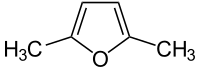
2,5-dimethylfuran
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,5-dimethylfuran | |||||||||||||||
| other names |
DMF (not unique) |
|||||||||||||||
| Molecular formula | C 6 H 8 O | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 96.13 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.903 g cm −3 |
|||||||||||||||
| Melting point |
−62 ° C |
|||||||||||||||
| boiling point |
92-93 ° C |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| Refractive index |
1.4363 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2,5-dimethylfuran (DMF; the abbreviation DMF is however common for the solvent dimethylformamide ) is a heteroaromatic organic - chemical compound . It consists of a furan backbone that is methylated at the carbon atoms in positions 2 and 5.
Extraction and presentation
The starting point for the production of 2,5-dimethylfuran is z. B. Biomass from sugar cane , especially fructose .
use
Dimethylfuran is seen as a potential biofuel that could replace ethanol . Dimethylfuran has a 40% higher energy density than ethanol, so it is comparable to gasoline . It is chemically stable and, unlike ethanol, does not absorb moisture from the atmosphere. It also has a lower tendency to evaporate.
Web links
- New fuel made from starch and cellulose . In: Welt Online, June 21, 2007
Individual evidence
- ↑ a b c d e f g Entry on 2,5-dimethylfuran in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-198.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet Version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Scientific Abbreviations, Acronyms, and Symbols, pp. 2-32.
- ^ A b Y. Román-Leshkov, CJ Barrett, ZY Liu, JA Dumesic: Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates . In: Nature . 447, No. 7147, June 2007, pp. 982-985. doi : 10.1038 / nature05923 . PMID 17581580 .

