Sugar acids
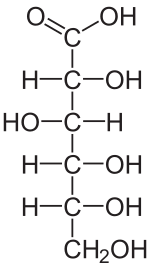
Fischer projection of D - gluconic , a counting of the sugar acids aldonic acid by the oxidation of D - glucose produced
Under sugar acids is meant poly hydroxy carboxylic acids formed by oxidation of simple sugars ( monosaccharides ) are formed. This case, in aldoses the aldehyde - group or the primary (terminal) alcohol group or at ketoses the primary alcohol group to a carboxy group oxidized.
- With mild oxidation , aldoses result in aldonic acids in which the aldehyde group is oxidized (e.g. D - glucose → D - gluconic acid ).
- If the primary alcohol group is oxidized in aldoses, uronic acids are obtained (e.g. D - glucose → D - glucuronic acid ).
- With more vigorous oxidation of aldoses, aldaric acids are formed (e.g. D - glucose → D - glucaric acid , the sugar acid in the narrower, technical sense) that carry two carboxy groups.
- Further examples: Threose → Threaric acid ( tartaric acid ), D - galactose → mucic acid , D- mannose → D- mannosacid
- Ketoaldonic acids result formally through oxidation of the primary alcohol group of ketoses, but technically through oxidation of a secondary alcohol group of aldonic acids (e.g. D- fructose → 2-oxo- D -gluconic acid).
Rules to remember about the oxidation products of monosaccharides
- Are in the Fischer projection the underlying monosaccharides o oxidized ben so on C1 , they are called O nsäuren ( aldonic ), for. B. gluconic acid.
- Where the underlying monosaccharides in the Fischer projection u nth o xidiert, so on the last C, they are called U r o nsäuren such. B. Glucuronic acid.
- Are in the Fischer projection the underlying monosaccharides, ie at C1 and C on the last (i. E., Up and oxidized below a lles is oxidized), they are called A rsäuren ( aldaric acids ), eg. B. glucaric acid.
properties
Sugar acids tend to form lactones . Many sugar acids act as metabolic products or are components of polymeric natural substances (eg. As the pectins , the glycosaminoglycans , of chondroitin sulfate , etc.).
swell
- Entry to sugar acids. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- Hans Beyer , Wolfgang Walter : Textbook of organic chemistry . 20th edition. Hirzel, Stuttgart 1984, ISBN 3-7776-0406-2 , p. 398.
Individual evidence
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 6: T-Z. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1988, ISBN 3-440-04516-1 , p. 4734.