Methyl cellulose
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
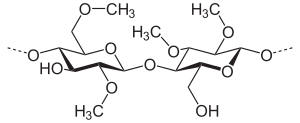 Section from a methyl cellulose polymer, degree of substitution 2 |
|||||||||
| General | |||||||||
| Surname | Methyl cellulose | ||||||||
| other names | |||||||||
| CAS number |
|
||||||||
| Monomers / partial structures | methylated cellobiose | ||||||||
| ATC code | |||||||||
| Brief description |
white to yellowish powder |
||||||||
| Drug information | |||||||||
| Drug class |
Laxative |
||||||||
| properties | |||||||||
| Physical state |
firmly |
||||||||
| safety instructions | |||||||||
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Methylcellulose (fachsprachlich, standard language: methyl cellulose ) is a chemical compound derived from cellulose is derived; chemically it is a cellulose ether with a meaning similar to carboxymethyl cellulose . It is a hydrophilic white powder and dissolves in cold (but not hot) water , forming a viscous solution or gel .
Methyl cellulose is the main ingredient in many wallpaper pastes and is sold under a variety of trade names. It is also used as a thickener and emulsifier in various food and cosmetic products and as a component of pharmaceuticals. Like cellulose, methyl cellulose is also indigestible, non-allergenic and non-toxic in the human organism.
Extraction and presentation
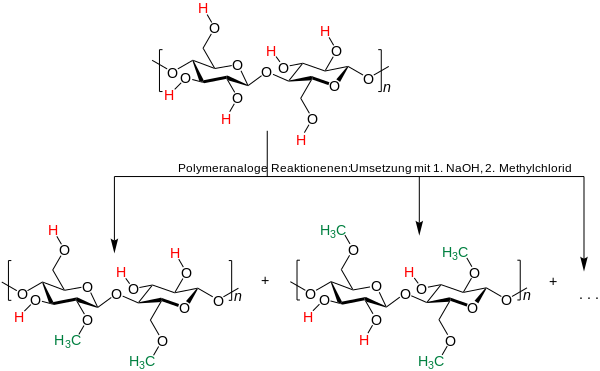
Methyl cellulose does not occur naturally, but is produced synthetically by placing cellulose in a hot, alkaline solution (a lye, for example sodium hydroxide ) and treating it with methyl halides .
Methyl cellulose is produced industrially by reacting cellulose that has been pretreated with an alkaline solution with methyl chloride at elevated temperature under pressure.
Since not all hydroxyl groups react in this reaction, mixtures are formed with different degrees of substitution . The degree of substitution of the individual cellobiose (ß1,4-glucose) building blocks within a polymer can also vary.
Chemical properties
Chemically, methyl cellulose is a methyl ether of cellulose and is created by replacing hydrogen atoms in the hydroxyl groups with methyl groups .
The methyl celluloses differ depending on the number of substituted hydroxyl groups. Cellulose is a chain molecule made up of numerous glucose molecules, each glucose unit has three hydroxyl groups. Different methyl celluloses can be described by their degree of substitution (DS), the average number of replaced hydroxyl groups per glucose unit. The theoretical maximum is 3.0, typical values are in the range from 1.3 to 2.6.
Methyl cellulose dissolves in cold water. The solubility decreases with increasing DS value because the polar hydroxyl groups are replaced by non-polar ether groups. Low-substituted methyl celluloses with a DS value <1 require an alkaline medium for solution. The substance is less soluble in hot water> 55 ° C than in cold. Therefore, heating a saturated solution of methyl cellulose causes it to precipitate. The temperature at which this effect occurs depends on the DS value. More highly substituted methyl celluloses are also soluble in organic solvents. Since the DS value of methyl cellulose is never uniform, there are always particles that cannot be dissolved. For this reason, mixtures of cellulose ethers are often produced which, in addition to methyl celluloses, also contain hydroxypropylmethyl cellulose (HPMC) and hydroxyethylmethyl cellulose (HEMC) or ethylmethyl cellulose (EMC).
Due to the different functional groups -OH and -OCH 3 , methyl cellulose has both hydrophilic and hydrophobic properties. As a result, it reduces the surface tension in solution and has an emulsifying effect in two-phase mixtures. In concrete mixes, it increases the water retention capacity and thus delays setting, so that the concrete components have more time for chemical reactions with the contained water. In ether mixtures, a film forms that can be regulated by adding formaldehyde . In methylcelluloses higher rates of these substitution film forming a thermoplastic polymer , which at temperatures of 120 to 190 ° C for water-soluble films extruded is.
use
Methyl cellulose is a compound that can be used in many ways. Methyl celluloses are used as thickeners, binders, adhesives, dispersants, suspending agents, emulsifiers, sedimentation agents, filter aids, flocculants, swelling agents, lubricants and water retention agents and as protective colloids and film formers. It's also often added to hair shampoos, toothpastes, and liquid soaps to create their signature viscosity.
Food and luxury foods
Methyl cellulose (additive number E 461 ) as well as other chemically modified derivatives of cellulose (E 463 to 469) are approved in the EU as a food additive , in the USA by 21CFR 182.1480 (FDA). In the food sector, they are often used as gelling, thickening and coating agents as well as emulsifiers and stabilizers in meat substitutes, ice cream, baked goods, cake creams, mayonnaise, instant products and frozen foods. Methyl cellulose films can be used as edible films.
In the beverage industry, they serve as binders for tobacco pieces in order to derive cigars leaves prepare for rolling cigars. These must have a share of more than 75% tobacco, the rest are binders.
medicine
Aqueous solutions of methyl cellulose are used as eye drops to treat dry eyes ("artificial tear fluid ").
After oral administration, methyl cellulose is not absorbed through the intestine, it binds large amounts of water and therefore has a laxative effect . Due to its swelling effect, it can be used as a laxative (laxative) to treat constipation , but this application is of secondary importance and rather uncommon in Germany.
In conventional radiology , methyl cellulose solution is used as a so-called negative contrast medium . The film is exposed more strongly at the points where the methyl cellulose has accumulated (it appears black on the X-ray ). It is mainly used to depict the small intestine.
pharmacy
In addition to its use as a medicinal substance, methyl cellulose is widely used as a pharmaceutical excipient in drug production: for example, to increase the viscosity in liquid pharmaceutical preparations , to stabilize emulsions and suspensions, as a gel former in creams and gels , as a binder for tablet granules, as a builder and disintegrant for tablets and as Film former for coated tablets.
According to the characterization in the European Pharmacopoeia, pharmaceutical grades of methyl cellulose contain a methoxy group content of 26% to 33%, which equates to a degree of substitution of about 1.6 to 2.0. The average molar mass is 10,000 to 220,000 grams per mole, corresponding to a degree of polymerization of approximately 50 to 1000. The various types of pharmaceutically used methyl cellulose are to be identified by specifying the viscosity of an aqueous solution at the prescribed concentration. Pharmaceutical grade methyl cellulose dissolves in cold water to form a colloidal solution ; it is practically insoluble in hot water, in acetone , anhydrous ethanol and toluene . The pH of a 1% solution is between 5.0 and 8.0. There are a number of incompatibilities with other substances that can lead to precipitation ("clumping") of methyl cellulose.
In ophthalmic preparations methylcellulose is employed in concentrations of 0.5-1.0%.
Technical and other use
Methyl cellulose is the main component of many wallpaper pastes and can be used as a mild adhesive that can be washed off with water. For example, it can be used to fixate delicate works of art.
Methyl cellulose is used on a large scale in the production of building materials. For example, it is added to dry mortar mixes in order to improve the properties of the mortar, such as water retention, viscosity and adhesion to the surfaces. It is also used as an additive in plaster mixes and putty substances for the same reasons . In wall and textile paints as well as in inks , methyl cellulose is used as a thickener and in cleaning agents and cosmetics as an emulsifier and stabilizer. Further uses are as a seed coating in the agricultural industry and as an additive to stabilize the monomer solution in the polymerization of vinyl chloride to polyvinyl chloride (PVC).
The slimy, sticky appearance of a suitable mixture of methyl cellulose with water, in addition to its non-toxic, non-allergenic properties, makes it a popular substance in special effects in photography and television where mud or slimy substances are needed. In the movie Ghostbusters, for example, the sticky substance of some ghosts was mostly a concentrated aqueous solution of methyl cellulose. Methyl cellulose is also widely used in the pornographic industry to simulate semen .
Like agar, methyl cellulose is used as a raw material in microbiological culture media, for example to study the reproduction of bacteria .
Individual evidence
- ↑ Entry on E 461: Methyl cellulose in the European database for food additives, accessed on August 6, 2020.
- ↑ Entry on METHYLCELLULOSE in the CosIng database of the EU Commission, accessed on August 6, 2020.
- ↑ a b c Entry on methyl cellulose. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ a b Data sheet methyl cellulose (PDF) from Carl Roth , accessed on December 14, 2010.
- ↑ Article methylcellulose in ChemgaPedia .
- ↑ DS of cellulose ether types ( Memento of the original from April 18, 2016 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Zusatzstoffe-online.de .
- ↑ a b c Keyword methyl cellulose . In: Hans Zoebelein (Ed.): Dictionary of Renewable Resources. 2nd edition, Wiley-VCH, Weinheim and New York 1996; Pages 189-190. ISBN 3-527-30114-3 .
- ↑ Keyword Tobacco Industry . In: Hans Zoebelein (Ed.): Dictionary of Renewable Resources. 2nd edition, Wiley-VCH, Weinheim and New York 1996; Pages 306-307. ISBN 3-527-30114-3 .
- ↑ a b c d Handbook of Pharmaceutical Excipients, 5th Edition (2006). Ray C. Rowe (Editor), Paul J. Sheskey (Editor), PJ Weller (Editor)
- ↑ Mutschler, Geisslinger, Kroemer, Schäfer-Korting, Mutschler drug effects , 9th edition, 2008, ISBN 380471952X .
- ↑ Drug information system at PharmNet.Bund .
- ↑ Methylcellulose In: Pschyrembel Clinical Dictionary. 257th edition, de Gruyter Verlag Berlin 1994. ISBN 3-11-012692-3
- Jump up the sluggish bowel , in: Pharmazeutische Zeitung , Edition 13, 2008.
- ↑ Commentary on the European Pharmacopoeia 6.1, loose-leaf collection, 31st volume 2009.
- ↑ European Pharmacopoeia 6th edition, basic work 2008, 6.0 / 0345.