HIV
| Human immunodeficiency virus | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

HI viruses in the TEM |
||||||||||||||||||||
| Systematics | ||||||||||||||||||||
|
||||||||||||||||||||
| Taxonomic characteristics | ||||||||||||||||||||
|
||||||||||||||||||||
| Scientific name | ||||||||||||||||||||
| Human immunodeficiency virus | ||||||||||||||||||||
| Short name | ||||||||||||||||||||
| HIV-1, HIV-2 | ||||||||||||||||||||
| Left | ||||||||||||||||||||
|
The human immunodeficiency virus ( scientifically human immunodeficiency virus ), mostly abbreviated as HIV (also HI virus ) or also referred to as human immunodeficiency virus or human immunodeficiency virus , is an enveloped virus belonging to the retrovirus family and to the genus Heard of lentiviruses . An untreated HIV infection leads to a long differently, usually several years symptom free latency period usually to AIDS (English acquired immunodeficiency syndrome , acquired immune deficiency syndrome ' ).
The spread of HIV has developed into a pandemic since the early 1980s , which the United Nations Joint Program on HIV / AIDS (UNAIDS) estimates has claimed around 39 million lives to date.
At the end of 2014, an estimated 36.9 million people worldwide were infected with HIV, with an even distribution across both sexes. At the end of 2018, around 87,900 people were living with an HIV infection in Germany, 80% of them men. In 2011 there were around 18,000 infected people in Austria , 5200 of whom were women. In 2013 there were around 20,000 HIV-infected people in Switzerland , 6100 of them women.
Structure and structure of the HI virus
HIV is one of the complex retroviruses , which means that the viruses have, in addition to the canonical retroviral genes gag, pol and env, further regulatory and accessory reading frames , namely in HIV-1 tat, rev, vif, vpu, vpr and nef.
The virus particle (virion) has a diameter of about 100 to 120 nanometers and is therefore larger than average for a virus, but significantly smaller than z. B. Erythrocytes (diameter 7500 nanometers). HIV is surrounded by a lipid bilayer that was separated from the human host cell during budding . Accordingly, various membrane proteins of the host cell are located in the virus envelope. Also embedded in this shell are about 10 to 15 so-called spikes per virion , about ten nanometers in size env glycoprotein complexes ; the density of the spikes is therefore quite low, as there would be space for 73 ± 25 of these processes on the surface of an HIV particle. An HIV spike consists of two subunits: the three molecules of the external surface (English surface ) glycoprotein gp120 are non- covalently to three molecules of the transmembrane envelope (English envelope ) glycoprotein gp41 bound. Gp120 is essential for the virus to bind to the CD4 receptors of the target cells. Since the envelope of the HIV virus from the membrane is formed of the host cell, are also in their various proteins of the host cell , for example, HLA class I and II molecules as well as adhesion proteins .
The gag-encoded matrix proteins p17 are associated with the inside of the membrane . Inside the virion is the virus capsid , which is made up of the capsid proteins encoded by gag. The capsid protein p24 can be detected as an antigen in fourth generation HIV tests . In the capsid, associated with the gag-coded nucleocapsid proteins, the viral genome (9.2 kb ) is found in the form of two copies of the single-stranded RNA. The task of the nucleocapsid proteins is to protect the RNA from degradation after it has penetrated the host cell. The capsid also contains the enzymes reverse transcriptase (RT), integrase and some of the accessory proteins. The protease plays a key role in particle formation and is therefore found throughout the virus particle.
history
HIV is the name recommended by the International Committee on Taxonomy of Viruses (ICTV) in 1986. It replaces the former names such as lymphadenopathy-associated virus (LAV), human T-cell leukemia virus III (HTLV-III) or AIDS-associated retrovirus (ARV).
HIV type 1 was first described in 1983 by Luc Montagnier and Françoise Barré-Sinoussi from the Pasteur Institute in Paris.
In the same issue of the journal Science , Robert Gallo , director of the tumor virus laboratory at the NIH in Bethesda , also published the discovery of a virus that he believes could cause AIDS. In this publication, however, he described the isolation of human T-cell leukemia viruses type I (HTLV-1; a short time later also HTLV-2 ) in AIDS patients who happened to be present in his samples alongside the HI virus, and did not isolate the HI virus until about a year later. In a press conference with then United States Secretary of Health Margaret Heckler , on April 23, 1984, he publicly announced that AIDS was caused by a virus he had discovered.
Both Montagnier and Gallo each claimed the first discovery for themselves. This was followed by years of legal dispute, including the patent for the newly developed HIV test. In 1986 HIV-2 was discovered.
The two researchers Françoise Barré-Sinoussi and Luc Montagnier were awarded the Nobel Prize in Medicine in 2008 for their discovery of the HI virus . The fact that Gallo was not taken into account met with some criticism from the virology community.
In May 2005, an international research team was able to prove for the first time that the origin of HIV lies in monkeys. The research team took 446 faecal samples from wild chimpanzees in the wilderness of Central African Cameroon. Several samples had antibodies against simian immunodeficiency virus (shortly SIV ; English simian immunodeficiency virus ) that chimpanzee version of the HIV virus, as the team in the US journal Science published. Twelve samples were almost identical to HIV-1 in humans. The team emphasized that the antibodies had previously only been detected in captive chimpanzees. However, the original source of the HIV virus is not the chimpanzees. They are said to have been infected with SIV or a precursor of this virus in other species of monkeys in western central Africa. Around the beginning of the 20th century, people became infected with SIV for the first time, which then mutated in their organisms into HIV, which caused AIDS. The virus has already crossed the species boundary at least twice , namely from apes to great apes and then to humans. How the virus was transmitted to humans is unclear. It is believed that hunters who hunted and ate monkeys were first infected with the virus, for example through small open wounds.
Another hypothesis was that a vaccine against poliomyelitis (polio) had been contaminated in 1959 by monkeys carrying the virus (see also the controversy over the development of AIDS ). According to this thesis, chimpanzee kidneys were used to multiply the vaccine in the former Belgian Congo and then hundreds of thousands of people were vaccinated by oral vaccination, whereby SIV was transmitted to humans and mutated into HIV. However, an analysis of the mutations showed that there was a 95 percent probability that the origin of the strain HIV-1 was before 1930. In February 2000 a sample of the oral vaccinations was found and examined. There were no traces of HIV or SIV in it.
The oldest infected person, whose HIV infection has been confirmed by blood samples, comes from the Belgian Congo and dates back to 1959. In the first publication of this serum sample, however, it was stated that the origin of the sample had not been established with certainty, and so it is It is possible that a person was infected for the first time in what was then French Equatorial Africa . A DNA paraffin sample of almost the same age from 1960, which also comes from the Belgian Congo, is genetically distinct from the first sample, which indicates that HIV was present in Africa long before 1960. Using statistical analyzes (so-called molecular clock ), the time window for the first occurrence can be narrowed down to the years between 1902 and 1921 with a high degree of probability. During that time, the African aborigines who u. a. served as workers and carriers for raw materials, treated by the Belgian colonial rulers in the Congo against sleeping sickness . Syringes that were not disinfected and therefore infected with HIV were used for several people. In addition to the poor medical and hygienic conditions, there were newly introduced diseases (including syphilis from Europe), which further weakened the population and made them vulnerable. The carrier columns could also have contributed to bringing the HI virus from the interior of the country to the coast, so that it could be spread further from there.
When HIV first appeared in the USA, i.e. at the starting point of the AIDS pandemic, has long been the subject of research with various, sometimes controversial results. AIDS-like symptoms were observed in the patient Robert Rayford as early as 1968 and later identified as HIV. However, it could not be clearly proven that this was the HIV circulating today. According to a current study from 2016, the virus (HIV-1 subtype B) is said to have traveled from Africa to Haiti in 1967 and from there to the USA around 1971, whereby it has been proven that Gaëtan Dugas , who had long been considered patient Zero , did not may have been the first infected person in North America. The information relates to phylogenetic extrapolations. The earliest serological evidence is found in samples from San Francisco and New York City, each from 1978 onwards.
The thesis of an unnatural HIV development, classified as a conspiracy theory , was created by the KGB in the course of the infection operation and given a presumably scientific background by Jakob Segal in Berlin in the 1980s.
Classification and system
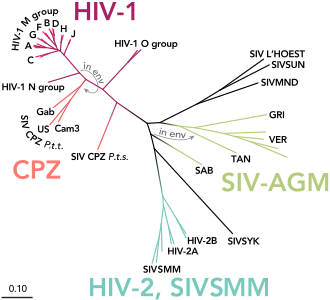
Two different types of HIV virus are known to date, which are referred to as HIV-1 and HIV-2. The homology between HIV-1 and HIV-2 is only about 45 to 50 percent at the amino acid sequence level . They can be further divided into subtypes, some of which occur with different frequencies in different regions of the world. In Central Europe, for example, subtype B from group M of HIV-1 is most common, especially among homosexuals and injecting drug users . HIV-1, which is more common overall, and HIV-2 are fundamentally similar in terms of the clinical course of infection and pathogenic properties, even if the infection with HIV-2 is likely to be slower overall. The two strains look the same under the electron microscope , but differ in the molar mass of the proteins and in the arrangement and nucleotide sequence of the genes . HIV-1 and HIV-2 arose from different types of the SI viruses found in certain species of monkeys.
HIV-1 is divided into four groups called M, N, O, and P. M stands for engl. major group 'main group' and is the most common, the O group was named after outlier 'special case' and the N of group N stands for new 'new' . Group M of HIV-1 includes more than 90 percent of all HIV infections and is responsible for the infection of around 60 million people worldwide (as of 2010). This group, in turn, is divided into nine subtypes, labeled A, B, C, D, F, G, H, J, and K. The most common are subtypes B (mainly found in North America and Europe), A and D (mainly in Africa) and C (mainly in Africa and Asia). A co-infection with different subtypes can lead to recombinant forms occur, the circulating recombinant forms (CRFs) can be mentioned. The classification of the HIV strains is correspondingly complex and not yet completed.
The HIV-1 group O seems to be distributed almost exclusively in West Africa , while the newly discovered groups N and P viruses have so far only been found in a few people.
HIV-1 was originally transmitted to humans from SIV infected chimpanzees and gorillas . For example, it was possible to prove that groups M and N came from chimpanzees, while HIV-1 P was transmitted from gorillas to humans. Whether HIV-1 O originally comes from SIV-infected chimpanzees or gorillas has not yet been conclusively clarified. All four HIV-1 groups (M, N, O and P) are due to four independent zoonoses (i.e., animal-to-human transmission), with only group M having reached the proportions of a pandemic . The reason for the higher infectivity of the HIV-1-M strain is based, among other things, on specific properties of the viral protein vpu of the M group, which help to overcome two infection barriers: On the one hand, the antiviral factor tetherin is effectively switched off and on human cells on the other hand, the CD4 receptor is broken down.
Epidemiology
Worldwide
The global HIV prevalence (disease frequency) among adults aged 15 to 49 years was 0.8 percent in 2010. For Central and Western Europe it was 0.2 percent. In sub-Saharan Africa (5.0 percent) and the Caribbean (0.9 percent) it was above average. In individual countries, such as Swaziland , Botswana or Lesotho , around a quarter of 15 to 49 year olds are infected with the HI virus. The HIV prevalence in 2010 was well below average in the regions of East Asia (0.1 percent) and in North Africa and the Middle East (0.2 percent). In Russia , the number of people infected with HIV has doubled within five years, around 1.2 million Russians are infected with the virus. This corresponds to about one percent of the population.
In 2014, around 1.2 million people worldwide died as a result of HIV infection. The number of known new infections has been falling steadily since 1997 and was two million people in 2014. The number of AIDS deaths has also been falling since 2005.
In Germany
For 2018, the number of new infections in Germany and among people of German origin who were infected abroad was estimated by the Robert Koch Institute at 2,400. There has been a slow, steady decline in the number of new infections for about fifteen years, with an estimated 2,800 new infections in 2015. Of the newly infected, an estimated 2,000 are men and an estimated 440 are women. The route of infection was sex between men in 1,600 cases , with a sustained sharp decline (and another 2,200 new infections in 2013). A further 530 new infections occurred through heterosexual contact and 310 times through intravenous drug use and in fewer than ten cases through transmission from mother to child.
Of the people with an initial HIV diagnosis in 2018 (which does not match a new infection), an estimated 1,000 people already had an advanced immunodeficiency , and 460 an AIDS disease.
In 2018, an estimated 440 people died as a result of HIV infection, and an estimated 29,200 people in Germany have died of it since the beginning of the HIV epidemic.
For the end of 2018, the Robert Koch Institute assumed that 87,900 people were living with an HIV infection in Germany, of which 10,600 were not known to be infected. Of those infected, an estimated 70,600 were men and 17,300 women.
An estimated 71,400 people with known HIV infection received antiretroviral therapy in 2018, which means treatment coverage of 92%. Of these, the therapy was so successful in 95% that they were not infectious.
transmission
| Route of infection | Risk per 10,000 contacts with an infectious source |
In percent |
|---|---|---|
| Parenteral | ||
| Blood transfusion | 9,250 | 92.50% |
| Drug injection with a used needle | 63 | 0.63% |
| Needle stick through the skin | 23 | 0.23% |
| Sexual (unprotected intercourse) | ||
| Anal intercourse, receiving partner | 138 | 1.38% |
| Vaginal intercourse, receiving partner | 8th | 0.08% |
| Anal intercourse, introductory partner | 11 | 0.11% |
| Vaginal intercourse, introductory partner | 4th | 0.04% |
| Oral sex | low 1 | - |
|
1 Cases of HIV transmission through oral sex have been scientifically documented but are rare. A precise estimate of the risk is not available due to the poor data situation. |
||
The HI virus is transmitted through contact with the body fluids of blood , semen , vaginal secretions as well as breast milk and cerebrospinal fluid . Potential entry points are fresh, still bleeding wounds and mucous membranes (especially conjunctiva , vaginal and anal mucosa) or insufficiently horny, easily vulnerable areas of the outer skin ( glans , inside of the penile foreskin , anus ). The most common route of infection is anal or vaginal intercourse without the use of condoms . The use of contaminated syringes during intravenous drug consumption is another common route of infection. Oral intercourse is considered to be far less infectious, since the healthy oral mucosa is much more resistant than other mucous membranes. An infection is only possible during oral sex if semen or menstrual blood reaches the oral mucosa. If vaginal fluid is absorbed without blood, the amount of virus is insufficient for infection. Oral ingestion of pre-ejaculate does not pose a risk if the oral mucosa is intact. Homosexual men are considered a risk group, since anal intercourse, which carries a significantly higher risk of infection than vaginal intercourse, is more common in them than in the heterosexual group . How high the risk is during sexual intercourse depends primarily on the virus concentration ( viral load ) in the body fluid that is transferred (e.g. blood, semen or vaginal secretions). The viral load is particularly high in the first few weeks after infection, before sufficient antibodies have formed, but then initially decreases and increases again in the later stages of the disease.
As has been shown in a study, the risk of infection for circumcised men is slightly lower. According to the most common assumption, circumcision leaves a smaller area of attack for the virus through the removal of the foreskin, although the original article already states that it could also be due to a less pronounced risk behavior of the target group. For this reason, the World Health Organization (WHO) recommended circumcision to its member countries in 2007 as a preventive measure to contain HIV / AIDS, for which it was criticized by experts. In fact, there are very few studies that have shown the benefits of circumcision. These studies are based e.g. T. on a very small sample. The view, which has meanwhile become widespread through the recommendation of the WHO, that circumcision could effectively reduce the risk of infection can therefore at least be questioned. Rather, it is feared that circumcised men believe they are in a false sense of security and take a higher risk.
Blood transfusions are also a possible source of infection: the risk of infection for the recipient in the event of a transfusion with HIV-contaminated blood is estimated at 90%. In the early 1980s, for example, various blood scandals broke out in many countries . Because of the routine tests for HIV antibodies in blood donors introduced in 1985, this possibility of infection is hardly of any significance today . Since in individual cases up to three months can elapse between the infection of the donor and the detection of antibodies in the HIV test ( diagnostic gap ), since the beginning of 2002 all German blood donations have also been tested for the presence of the virus using the polymerase chain reaction (PCR) . The Robert Koch Institute estimates the risk of becoming infected with HIV when donating blood in Germany to be less than 1: 5 million.
The risk of infection to a child by an HIV-infected mother during pregnancy or childbirth without treatment is estimated to be 15 to 30%. Transmission of the virus while breastfeeding is also possible. If the mother is known to be infected with HIV, the risk of transmission to the child can be reduced to less than one percent by giving antiretroviral drugs (to the mother before and after the birth), giving birth by caesarean section and not breastfeeding the child become.
The so-called CHAT survey study by the Swiss Federal Office of Public Health (FOPH) - a follow-up survey of people who received positive HIV tests in the course of a year - showed that 49% of all newly infected people received the infection from their steadfast sexual partner; 38% were infected by a known but not permanent casual partner. Ten percent of the newly infected people knew beforehand that their partner was HIV positive. If someone has been deliberately infected by their already infected partner, this is known as Pozzen . Only 13% of heterosexuals became infected through anonymous sexual encounters. In homosexuals, infections from steadfast partners played a smaller role - anonymous sexual contacts made up 26% of infections.
French kisses are not considered to be a risk of HIV infection. Although the possibility of infection appears theoretically conceivable if bleeding wounds , for example injuries to the gums, are present in the mouth, there is no documented case of this transmission route anywhere in the world.
The HIV concentration in tears , sweat and saliva is insufficient for infection. Furthermore, HIV is not transmitted through droplets or through food or drinking water. In addition, the AIDS epidemiology makes infection from insect bites extremely unlikely.
People who have been exposed to an acute risk of infection should consult a doctor as soon as possible (ideally within hours) for advice and, if necessary, post-exposure prophylaxis (PEP). After 48 or 72 hours, drug-based post-exposure prophylaxis is no longer considered useful.
With regard to the probability of infection, the treatment indications and therapy, see in detail under AIDS .
HIV multiplication cycle
Follicular T helper cells as an HIV reservoir
In order to multiply, the virus needs host cells that have the CD4 receptor on the surface. These are mainly CD4-carrying T helper cells (CD4 + cells). The main reservoir for human immunodeficiency viruses are the follicular T helper cells in the body's lymph follicles , which make up around two percent of CD4 + cells. T helper cells support other white blood cells in immunobiological processes, such as the maturation of B lymphocytes into plasma and memory cells or the activation of cytotoxic T lymphocytes and macrophages . In addition to T lymphocytes, monocytes , macrophages and dendritic cells also have CD4 receptors. Latently infected, dormant CD4 + T cells ( T memory cells ) represent long-lasting reservoirs for HIV and are the reason why HIV has not yet been eradicated despite effective antiretroviral drugs and recurrences occur again and again after treatment is discontinued .
Fusion with the host cell

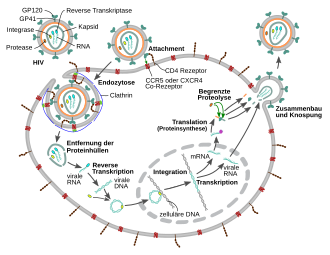
1. The surface protein gp120 binds to the CD4 receptors .
2. Conformational change in. Gp120 enables a subsequent interaction with CCR5.
3. The distal tips of gp41 penetrate the cell membrane.
4. The binding leads to a conformational change in the transmembrane protein gp41 (“snap spring mechanism”). This process fuses the viral and cellular membranes together.
In order to be able to fuse with the cell membrane of the host cell , the surface proteins gp120 bind to the CD4 receptors . The binding results in a conformational change in the transmembrane protein gp41, a mechanism that is similar to a “snap spring” or a “mousetrap”.
In addition to the CD4 receptors, other co-receptors are involved in the binding of the HI virus to white blood cells: the chemokine receptors CCR5 on monocyte cells and CXCR4 on T cells are involved in the binding. The different levels of these receptors influence the likelihood of infection and the course of the HIV infection. Molecules that block the CCR5 receptors belong to the drug group of entry inhibitors , but play a subordinate role in current HIV therapies.
Likewise, people who are homozygous with the so-called Delta-32 mutation of the CCR5 co-receptor gene are more difficult to infect with HIV. This applies to around one percent of the population in Europe. To a lesser extent, this also applies to mutations in the CCR2 gene. People with HLA B27 / B57 (see Human Leukocyte Antigen ) show a slower course of the disease.
Incorporation of the HIV genome into the host cell
To multiply, HIV builds its genetic material, which it has in the form of an RNA genome, into the double-stranded DNA of the host cell 's genome after what is known as reverse transcription . The conversion of viral RNA into proviral DNA in the host cell's cytoplasm by the enzyme reverse transcriptase is a crucial step in the retrovirus reproductive cycle. Since the reverse transcriptase of retro and hepadnaviruses is very different from other reverse transcriptases such as human telomerase, it is an important target of therapeutic intervention and is the starting point for two pharmacological drug classes.
After reverse transcription and transport into the cell nucleus, the virus genome is integrated into the human genome by another viral enzyme, the integrase . The viral DNA is read before integration, and the viral proteins are also formed. Accordingly, the HIV-DNA exists as an integrated and a non-integrated form. Circular forms of HIV DNA also exist.
The viral genome, now available as an integrated provirus , has a characteristic structure, with the coding regions being flanked on both sides by identical regulatory sequences that were generated in the course of reverse transcription, the so-called LTRs . The promoter , under the control of which the various mRNAs are transcribed , is located in the area of the LTR and is activated by the viral protein TAT . An unspliced RNA serves as the viral genome for the next generation of HI viruses and as mRNA for the translation of a Gag ( group-specific antigen ) and by means of a shift in the reading frame of a Gag - Pro - Pol precursor protein, which occurs in one out of 20 cases . Spliced RNAs code for the envelope protein Env and the other proteins which are also located in the 3 'region. HIV codes for 16 proteins.
Morphogenesis then follows , which means that the viral components such as Gag, Pro-pol and Env precursor proteins as well as the RNA come together through various interactions and form initially immature virions that are pinched off the plasma membrane. Further maturation processes produce the mature virus particle, ready to infect the next cell. The maturation processes include, in particular, the cleavage of the precursor proteins - partly by the viral protease , partly by cellular enzymes - into their individual components, i.e. from Gag into matrix, capsid and nucleocapsid protein, Pol into protease, reverse transcriptase with RNase H and integrase as well Env in surface and transmembrane unit. The newly formed daughter virions leave the cell by budding . During assembly, the HIV becomes covered with the cellular protein cyclophilin A. One cyclophilin A binds to two hexamers of the capsid protein, thereby stabilizing the capsid and masking it from intracellular mechanisms of the innate immune response in macrophages and dendritic cells.
The virus in infected and resting CD4 + - T cells escapes the attack by antiviral drugs and the immune system . These “immune cells” are activated after contact with the antigen , for example in the context of a normal or an opportunistic infection . While the cell actually wants to take action against another pathogen, it instead begins to produce virus proteins and release new viruses. These then in turn infect other cells.
What makes the HI virus so extraordinarily viable is its adaptability or, better said, its high rate of evolution . For example, not even half as many new variants of the influenza virus (flu) develop around the world in the same time as the HIV virus in a single infected person.
Course of HIV infection

Untreated HIV infection usually has several stages. After an incubation period of around three to six weeks, an acute HIV infection usually occurs after infection. This is characterized by fever , heavy night sweats , fatigue, skin rashes, oral ulcerations or arthralgia (joint pain). Because of the similarity with flu-like infections, the acute HIV infection mostly goes undetected. However, an early diagnosis is important: it can not only prevent further infections of sexual partners. Initial studies on patients who received antiviral treatment during acute HIV infection and who discontinued therapy after some time showed that the patient's HIV-specific immune response could be strengthened. The acute infection rarely lasts more than four weeks.
In the following, usually several years latency phase , no serious physical symptoms occur. Changes in blood values and creeping lipodystrophy often go unnoticed by those infected with HIV. After that, there are often the first illnesses that can be traced back to a moderately weakened immune system, but are not yet considered AIDS-defining (CDC classification B, see AIDS ).
In addition to the symptoms caused by the weakening of the immune system, there are also other symptoms, such as changes in the structure of the heart muscle. Structural changes in the heart mainly occur when it is not possible to reduce the viral load adequately.
Destruction of CD4 helper cells
In the course of an HIV infection, among other things, CD4 + helper cells are continuously destroyed in various ways, which weakens the immune system. On the one hand, infected host cells can be eliminated directly. This happens either through membrane damage to the cell, which is caused by the entry and exit of viruses, or through proapoptotic proteins of the HI viruses and destructive information hybrids of RNA and DNA. On the other hand, infected cells are indirectly destroyed, which healthy cells of the immune system recognize as dangerous and are then switched off by them. In addition, uninfected T helper cells are also destroyed as collateral damage through contact with proteins such as p120. These proteins are produced when the HIV virus multiplies in the bloodstream. Following an acute HIV infection and following a virus-specific immune response , the body is usually able to replace most of the destroyed cells with the production of new cells over a period of several years.
Education of an immunodeficiency
If the HIV infection remains untreated, the number of CD4 + helper cells decreases continuously, and a median of nine to eleven years after the initial infection leads to a severe immune deficiency (<200 CD4 + cells / microlitre). This usually leads to AIDS-defining diseases ( CDC classification 3). These include opportunistic infections caused by viruses , bacteria , fungi or parasites , as well as other diseases such as Kaposi's sarcoma , malignant lymphoma , HIV encephalopathy and wasting syndrome . If left untreated, these usually lead to death after individually varying times. However, a severe immunodeficiency does not mean that AIDS will occur immediately. The longer you have a severe immune deficiency, the greater the chance of getting AIDS.
Genetic factors and resistance
The fact that individuals often have very different disease courses despite the same source of infection indicates a strong influence of host factors on the course of the infection. In addition to the development of the immune response, some genetic factors also seem to play a role. Various groups do not get AIDS, e.g. B. the long-term non-progressors (LTNPs, including the elite controllers ) and the highly exposed persistently seronegative (HEPS). The LTNP will not develop progressive disease, while the HEPS will not become infected with HIV.
Homozygous individuals with a genetic defect in the CCR5 receptor (CCR5delta32) are largely resistant to HIV infections.
This receptor serves as a co-receptor in the fusion of the virus with the host cell. Only a few individuals have been found who have an infection despite this receptor defect. They became infected with HIV viruses that use other co-receptors, such as the CXCR4 receptor on T cells. Homozygous gene carriers of this deletion make up about one percent of the population, heterozygous gene carriers about 20 percent. Although heterozygotes have significantly fewer CCR5 receptors, they can also become infected with HIV and hardly seem to have a longer mean survival time after infection.
Aside from mutations that confer complete resistance to HIV, there are also a number of genotypes that, while not protecting against HIV infection, are associated with slower disease progression and lower viral loads. Two different mechanisms have been identified:
- Carriers of certain alleles of the MHC -I proteins, in particular HLA-B * 5705 and / or HLA-B * 2705, show a slower progression of the infection compared to other people. Since MHC-I proteins bind viral proteins from inside the cell and thus indicate the infection of a cell, it is assumed that the variants mentioned are able to bind the proteins of HIV particularly efficiently. Therefore, HIV-infected T helper cells in these individuals are recognized and destroyed particularly quickly by cytotoxic T cells .
- After an HIV infection, the immune system begins to produce antibodies against HIV; however, due to the high mutation rate of HIV, these remain largely ineffective. However, some people produce antibodies that target a constant region of the gp120, which slows down the infection. Why these antibodies are only produced by certain people is unknown.
Tests for HIV infection
There are various ways of testing blood, semen, urine or even tissue for a possible infection with HIV. The virus can be detected either directly by the viral RNA or the antigen p24 (a protein of the HIV capsid ) or indirectly by the antibodies against HIV produced by the body .
Immunological test procedures
The serological test procedures, often incorrectly referred to colloquially as "AIDS tests", detect the antibodies against the virus formed by the human immune system. Modern fourth-generation screening tests (HIV-1/2 antibodies & p24 antigen combination tests) also detect the p24 antigen of the HIV-1 virus. Since p24 can already be detected in the early phase of the infection, when no antibodies have yet been formed, this shortens the diagnostic window and enables an earlier meaningful test result. From the 11th day of infection, a positive result is possible. A negative result only reliably rules out an infection if there was no possibility of infection 3 months before the test.
Automated immunassays are used as search tests in most routine laboratories and the classic ELISA test on microtiter plates is only used in isolated cases . In the event of a positive or borderline result in the immunassay, an immunoblot according to the Western blot principle follows as a confirmatory test . These two methods are always used one after the other: the immunassay is highly sensitive and therefore suitable for avoiding false negative results. The lower specificity of the screening test is accepted in order not to miss any positive samples at this stage of the diagnosis. The more specific immunoblot used for confirmation serves to rule out false positive results. Immunassays such as Western Blot are inexpensive tests and are highly accurate approx. Three months after a possible infection, but can be used earlier after a suspected infection, because the time for the formation of HIV antibodies is on average about 22 days. However, AIDS-Hilfe and the Robert Koch Institute recommend a waiting period of twelve weeks for a final test result from an immunassay . This does not apply to the fourth generation tests, which in addition to the antibodies detect the p24 antigen. These combined antibody-antigen screening tests provide a meaningful result after just six weeks.
A positive result in the screening test alone is not a reliable result of an HIV infection, which is why it is always used together with Immunoblot. If a positive result in the immunassay cannot be confirmed by means of immunoblot (which can only detect antibodies), an HIV-PCR must be carried out for direct pathogen detection, because the immunassay may only react to the p24 antigen of the virus while still no antibodies are present. If the immunassay can neither be confirmed by blot nor by PCR, it can be assumed that the immunassay has reacted “unspecifically”, ie. H. became positive for anything other than HIV infection. In rare cases, antibody tests can produce false positive results after recent acute illnesses, flu vaccinations and allergies.
PCR
The direct detection of viral nucleic acids ( ribonucleic acid (RNA)) by polymerase chain reaction (PCR) is the fastest, but also the most expensive method, which delivers reliable results just 15 days after infection. Aside from qualitative PCR, as used to diagnose acute HIV infection and in the blood donation system, quantitative PCR methods are an important tool for determining the viral load (number of viral RNAs per milliliter of blood plasma) in HIV-positive patients. to z. B. Monitor the success of antiretroviral therapy .
treatment
With antiretroviral therapy (ART) virus replication can be slowed down in the body and the onset of AIDS disease infection be delayed. From 1996 onwards, increasingly effective antiretroviral drugs came onto the market, which were initially used as a combination of three and later as a combination of two for the treatment of HIV infection. This highly active antiretroviral therapy (HAART), which is necessary for life, can almost completely prevent AIDS. In addition, the viral load drops in 95% of those treated to less than 200 virus copies per milliliter of blood and thus to such an extent that they are no longer infectious and the virus can thus be prevented from being passed on.
Therefore, the treatment guideline has been in effect since 2015 to treat every diagnosed HIV infection in Germany with antiretroviral. This happened in 2019 in 93% of known HIV-infected people.
prophylaxis
In order to prevent an HIV infection, the main focus was initially on protection from the three usual transmission routes: sexual intercourse, transmission through blood products and intravenous drug use .
For sexual intercourse , the use of condoms is still recommended as a “cornerstone” of the prevention of HIV and other sexually transmitted diseases . Occasionally, abstinence is also postulated, or at least the avoidance of high-risk behavior.
In the 1980s there was also a problem with the transmission of HIV through blood products such as plasma or blood transfusions , but also with the transmission of viral hepatitis . As a result, men who have sex with men have been excluded from donating blood and plasma for years . In the meantime, there are direct virus controls that have greatly reduced the risk of transmission.
In the European Union , a combination preparation of two modern antiretroviral agents ( emtricitabine and tenofovir ) was approved under the name Truvada, which, like generics, is indicated for occasional sporadic use by non-infected people, and the risk of infection during sexual intercourse with an HIV-infected person (whose Viral load is not already reduced by antiretroviral therapy) by a further 92%. Since September 1, 2019, the statutory health insurance companies in Germany have been assuming the costs for this pre-exposure prophylaxis . However, this “PrEP” treatment is not yet sufficiently well known and, according to German Aidshilfe, “the potential […] is far from exhausted”, especially since an estimated 10,600 people in Germany who are not aware of this infection are living with HIV.
Currently, the following points are being mentioned by the Robert Koch Institute and German Aids Aid in 2019 to further prevent virus transmission :
- Increasing the willingness to take HIV tests with low-threshold offers and free access for everyone ("expansion of the test offer")
- Allocation of clean disposable syringes and free access to drug substitution therapy even in prisons
- Access to HIV therapy for all infected people, including those without valid residence permits and also for HIV-positive drug users, of whom only 55% received antiretroviral therapy in 2016 (against 93% of all HIV-infected people)
- Increased use of PrEP and free access to it for all
Reporting requirement
In Germany, direct or indirect evidence of HIV does not have to be reported by name according to Section 7 (3) of the Infection Protection Act (IfSG).
In Switzerland, positive laboratory analytical findings on the HI virus must be reported in accordance with the Epidemics Act (EpG) in conjunction with the Epidemics Ordinance and Annex 3 of the EDI Ordinance on the reporting of observations of communicable diseases in humans .
See also
- Discrimination against people with HIV / AIDS
- HIV vaccine
- HIV / AIDS in Africa
- Highly active antiretroviral therapy
- Safe sex
literature
Guidelines
- S2k guideline for antiretroviral therapy of HIV infection of the German AIDS Society (DAIG). In: AWMF online (as of 2012)
- S2k guideline therapy and prophylaxis of opportunistic infections in HIV-infected patients of the German AIDS Society (DAIG). In: AWMF online (as of 2011)
- S1 guideline for the diagnosis and treatment of HIV-affected couples who wish to have children from the German AIDS Society (DAIG). In: AWMF online (as of 2008)
- S2k guideline German-Austrian recommendations on HIV therapy in pregnancy and in newborns exposed to HIV from the German AIDS Society (DAIG). In: AWMF online (as of 2011)
Others
- Christoph Benn, Sonja Weinreich: HIV and AIDS. A disease changes the world. Lembeck, Frankfurt am Main 2009, ISBN 978-3-87476-586-2 .
- Hans Jäger (Ed.): Entry Inhibitors. New forms of HIV therapy. Springer, Heidelberg 2008, ISBN 978-3-540-78357-2 .
- Niko Neye: Human Immunodeficiency Virus (HIV). A scientometric analysis . Free University of Berlin 2009 ( dissertation ).
- The scientific journal AIDS Reviews is published quarterly and publishes review articles dealing with the various aspects of HIV and AIDS.
Web links
German
- Information on HIV / AIDS. Robert Koch Institute
- Detailed and current information on the subject of HIV at hivbuch.de
- HIV diagnostics on laborlexikon.de
- Risk of infection with oral sex
- Opinion on the AIDS criticism. Robert Koch Institute
- DAGNÄ e. V. (guidelines and statements of the German Association of Resident Doctors in the Care of HIV-Infected)
Other languages
- HIV database (English)
- HIV InSite (English)
Individual evidence
- ↑ a b c d ICTV: ICTV Taxonomy history: Commelina yellow mottle virus , EC 51, Berlin, Germany, July 2019; Email ratification March 2020 (MSL # 35)
- ↑ a b c d e f Ulrich Marcus, Barbara Gunsenheimer-Bartmeyer, Christian Kollan, Viviane Bremer V: HIV Annual Report 2017/2018 . Epidemiological Bulletin 2019, issue 46 of November 14, 2019, pages 493–501, published by the Robert Koch Institute , doi: 10.25646 / 6411 , Link (PDF)
- ↑ Global Report (2012). (PDF; 1 MB) UNAIDS, 2013, accessed on June 13, 2015 (English).
- ↑ Switzerland HIV and AIDS estimates (2014). UNAIDS, 2014, accessed June 13, 2015 .
- ↑ P. Zhu et al .: Distribution and three-dimensional structure of AIDS virus envelope spikes . In: Nature . Volume 441, No. 7095, 2006, pp. 847-852. PMID 16728975 .
- ^ KH Roux, KA Taylor: AIDS virus envelope spike structure . In: Curr Opin Struct Biol . Volume 17, No. 2, 2007, pp. 244-252. PMID 17395457 .
- ^ J. Liu et al .: Molecular architecture of native HIV-1 gp120 trimers . In: Nature . Volume 455, No. 7209, 2008, pp. 109-113. PMID 18668044 .
- ↑ DC Chan, D. Fass u. a .: Core structure of gp41 from the HIV envelope glycoprotein. In: Cell. Volume 89, Number 2, April 1997, pp. 263-273, ISSN 0092-8674 , PMID 9108481 .
- ^ John Coffin et al .: What to call the AIDS virus? In: Nature. Volume 321, May 1, 1986, p. 10, doi: 10.1038 / 321010a0 .
- ↑ F. Barré-Sinoussi, JC Chermann et al .: Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). In: Science , Volume 220, Number 4599, May 1983, pp. 868-871, PMID 6189183 .
- ↑ RC Gallo, PS Sarin et al .: Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). In: Science , Volume 220, Number 4599, May 1983, pp. 865-867, PMID 6601823 .
- ^ ORF : When Robert Gallo did not discover the HI virus , April 2009
- ^ The Nobel Prize in Physiology or Medicine 2008. In: Internet pages of the Nobel Prize . The Nobel Foundation, accessed December 18, 2008 .
- ↑ J. Cohen, M. Enserink: Nobel Prize in Physiology or Medicine. HIV, HPV researchers honored, but one scientist is left out. In: Science. Volume 322, Number 5899, October 2008, pp. 174-175, ISSN 1095-9203 . doi: 10.1126 / science.322.5899.174 . PMID 18845715 .
- ^ S. Pincock: HIV discoverers awarded Nobel Prize for medicine. In: Lancet. Volume 372, Number 9647, October 2008, pp. 1373, ISSN 1474-547X . PMID 18946912 .
- ↑ C. Ballantyne: Nobel decision stirs viral dismay. In: Nature medicine. Volume 14, Number 11, November 2008, p. 1132, ISSN 1546-170X . doi: 10.1038 / nm1108-1132b . PMID 18989265 .
- ↑ a b c Aids - legacy of the colonial era. ARD , November 26, 2016, accessed on August 14, 2019 ( film online on YouTube ).
- ↑ Jan Osterkamp: How monkey immunodeficiency viruses suddenly conquered humans . In: Spektrum.de. 23 August 2019.
- ^ Edward Hooper: Aids and the Polio Vaccine. In: London Review of Books , Volume 25, No. 7, 2003.
- ↑ Edward Hooper: untruths, misrepresentations and spin: the dubious methods and tactics used by Stanley Plotkin's group in the "Origins of AIDS" debate . uow.edu.au, 2004; accessed on October 28, 2014.
- ↑ AIDS Origins . At: aidsorigins.com - Edward Hooper's website; accessed on October 28, 2014.
- ↑ B. Korber, M. Muldoon, J. Theiler et al .: Timing the origin of the HIV-1 pandemic. In: Programs and abstracts of the 7th Conference on Retroviruses and Opportunistic Infections. Abstract L5, January 30 - February 2, 2000.
- ^ Philippe Blancou, Jean-Pierre Vartanian et al .: Polio vaccine samples not linked to AIDS. In: Nature , Volume 410, pp. 1045-1046, doi: 10.1038 / 35074171 .
- ↑ Jörg Albrecht, Volker Stollorz: Vaccination without borders. In: FAZ.net . October 6, 2014, accessed December 27, 2014 .
- ↑ T. Zhu, BT Korber et al .: An African HIV-1 sequence from 1959 and implications for the origin of the epidemic. In: Nature , Volume 391, Number 6667, February 1998, pp. 594-597, doi: 10.1038 / 35400 . PMID 9468138 .
- ^ AJ Nahmias, J. Weiss, X. Yao et al .: Evidence for human infection with an HTLV III / LAV-like virus in Central Africa, 1959 . In: The Lancet . 327, No. 8492, June 1986, pp. 1279-1280. doi : 10.1016 / S0140-6736 (86) 91422-4 .
- ^ A b Michael Worobey et al .: Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960 . In: Nature . 455, October 2008, pp. 661-664. doi : 10.1038 / nature07390 .
- ↑ The history of the HI virus. Wissenschaft.de , June 27, 2008, accessed on September 12, 2019 .
- ↑ M. Elvin-Lewis, M. Witte, C. Witte, W. Cole, J. Davis: Systemic Chlamydial infection associated with generalized lymphedema and lymphangiosarcoma. In: Lymphology . Vol. 6, No. 3, Sep. 1973, pp. 113-121, ISSN 0024-7766 , PMID 4766275 .
- ^ RF Garry, MH Witte, AA Gottlieb, M. Elvin-Lewis, MS Gottlieb, CL Witte, SS Alexander, WR Cole, WL Drake: Documentation of an AIDS virus infection in the United States in 1968. In: JAMA: The Journal of the American Medical Association . Vol. 260, No. Oct. 14, 1988, pp. 2.085-2.087, PMID 3418874 .
- ^ M. Worobey, TD Watts, RA McKay, MA Suchard, T. Granade, DE Teuwen, BA Koblin, W. Heneine, P. Lemey, HW Jaffe: 1970s and 'Patient 0' HIV-1 genomes illuminate early HIV / AIDS history in North America. In: Nature. [Electronic publication before printing] October 2016, doi: 10.1038 / nature19827 , PMID 27783600 .
- ↑ Harold Jaffe, James Curran et al. a .: The acquired immunodeficiency syndrome in a cohort of homosexual men. A six-year follow-up study. In: Annals of Internal Medicine. 1985; 103 (2): pp. 210-214, doi: 10.7326 / 0003-4819-103-2-210
- ↑ Cladd Stevens, Edith Zang et al .: Human T-cell lymphotropic virus type III infection in a cohort of homosexual men in New York City. In: JAMA , 1986; 255 (16), pp. 2167-2172. doi: 10.1001 / jama.1986.03370160065028
- ↑ a b J. C. Plantier et al .: A new human immunodeficiency virus derived from gorillas . In: Nat. Med. . 15, No. 8, 2009, pp. 871-872. PMID 19648927 .
- ↑ Introduction to HIV types, groups and subtypes . AIDS charity AVERT. Retrieved April 23, 2010.
- ↑ a b c d F. Kirchhoff: "Optimal" adaptation of pandemic HIV-1 strains to humans . In: BIOspectrum . 2, 2010, pp. 144-148.
- ^ BH Hahn et al .: AIDS as a zoonosis: scientific and public health implications . In: Science . 287, No. 5453, 2000, pp. 607-614. PMID 10649986 .
- ↑ D. Sauter et al .: Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains . In: Cell Host Microbe . 6, No. 5, 2009, pp. 409-421. PMID 19917496 .
- ↑ "Optimal" adaptation of HIV-1 to the human host a prerequisite for the effective spread of the AIDS pandemic? . Ulm University . Archived from the original on January 15, 2016. Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Retrieved June 24, 2010.
- ↑ a b World AIDS Day report 2011. (PDF; 618 kB) UNAIDS, accessed on September 2, 2012 (English).
- ↑ kna: The number of people infected with HIV in Russia almost doubled in five years. In: aerzteblatt.de . November 28, 2012, accessed December 27, 2014 .
- ^ How AIDS changed everything. (PDF; 12.3 MB) UNAIDS, 2015, accessed on April 6, 2016 (English).
- ↑ Figures on the mean transmittability of HIV, per file. CDC , accessed on November 17, 2016 (English).
- ↑ Cornelia Dick-Pfaff: HI viruses also penetrate healthy vaginal mucous membranes. In: Wissenschaft-aktuell.de. December 17, 2008, accessed December 18, 2008 .
- ↑ Pietro Vernazza: Oral sex without rubber: How high is the risk of HIV? In: Clinic for Infectious Diseases / Hospital Hygiene, Kantonsspital St. Gallen. June 10, 2002, accessed January 31, 2014 .
- ↑ HIVreport - Lusttropfen. (PDF) (No longer available online.) Deutsche AIDShilfe, archived from the original on April 18, 2016 ; Retrieved April 6, 2016 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Figures on the mean transmission of HIV, per file. CDC , accessed July 1, 2009 .
- ↑ Cornelia Pfaff: Circumcision protects against HIV. In: Internet pages from Bild der Wissenschaft . March 26, 2004, accessed September 28, 2019 .
- ↑ Robert C. Bollinger: Male circumcision and risk of HIV-1 and other sexually transmitted infections in India. In: The Lancet . March 27, 2004, accessed December 5, 2011 .
- ↑ Can circumcision reduce the risk of HIV infection? HIV Transmission - Frequently Asked Questions. On: gib-aids-keine-chance.de ; accessed on January 15, 2015.
- ^ M. Hilgartner: AIDS in the transfusion recipient . In: Pediatr Clin North Am . 38, No. 1, 1991, pp. 121-131. PMID 1987513 .
- ↑ Irja Most: How safe are German clinics? In: tagesspiegel.de . February 29, 2008, accessed December 27, 2014 .
- ↑ Blood Safety: Frequently Asked Questions. Robert Koch Institute , May 19, 2014, accessed April 7, 2016 .
- ↑ Breastfeeding: Prophylaxis halves the risk of HIV . In: aerztezeitung.de.
- ^ RKI guide for doctors: HIV / AIDS. Robert Koch Institute , March 2011, accessed January 30, 2014 .
- ↑ CHAT Survey Study
- ↑ Andrea Fischer: Many get HIV from their partner. (No longer available online.) In: Tages-Anzeiger . May 2, 2006, archived from the original on May 30, 2011 ; Retrieved December 18, 2008 .
- ↑ HIV transmission and risk of AIDS. (No longer available online.) Federal Center for Health Education , 2015, archived from the original on June 6, 2016 ; Retrieved April 6, 2016 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ RKI guide for doctors: HIV / AIDS. Robert Koch Institute , August 2015, accessed April 6, 2016 .
- ↑ PG Jupp, SF Lyons: Experimental assessment of bedbugs (Cimex lectularius and Cimex hemipterus) and mosquitoes (Aedes aegypti formosus) as vectors of human immunodeficiency virus. In: AIDS , Sep. 1987, Volume 1, No. 3, pp. 171-174, PMID 2450552 : “ […] unlikely to occur in bedbugs under natural conditions. ”
- ↑ PA Webb, CM Happ, GO Maupin et al .: Potential for insect transmission of HIV: experimental exposure of Cimex hemipterus and Toxorhynchites amboinensis to human immunodeficiency virus. In: The Journal of infectious diseases (J Infect Dis), December 1989, Volume 160, Number 6, pp. 970-977. " [...] The persistence of HIV in an insect or on its mouthparts is one of many factors necessary for mechanical transmission in nature. The risk of insect transmission of HIV appears to be extremely low or nonexistent. ”
- ↑ Pest expert Karolin Bauer-Dubau quoted in Claudia Fromme: Bugs, the enemy in my bed . In: Süddeutsche Zeitung . February 25, 2007. About bed bugs and hair follicle mites. In: CME premium training fd med. Practice , No. 2, 2010, Springer.
- ^ CB Wilen, JC Tilton, RW Doms: HIV: cell binding and entry. In: Cold Spring Harbor perspectives in medicine. Volume 2, number 8, August 2012, p., Doi: 10.1101 / cshperspect.a006866 , PMID 22908191 , PMC 3405824 (free full text).
- ↑ Researchers discover a long-sought hiding place for the HI virus. Der Standard, December 18, 2012, accessed December 19, 2012 .
- ↑ Giuseppe Pantaleo et al .: Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. Journal of Experimental Medicine , accessed December 19, 2012 .
- ↑ MJ Buzon, H. Sun et al .: HIV-1 persistence in CD4 + T cells with stem cell-like properties. In: Nature medicine , Volume 20, Number 2, February 2014, pp. 139-142, ISSN 1546-170X . doi: 10.1038 / nm.3445 . PMID 24412925 . PMC 3959167 (free full text).
- ↑ JB Dinoso, SY Kim, AM Wiegand: Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy . In: PNAS . 106, No. 23, June 2009, pp. 9403-9408. doi : 10.1073 / pnas.0903107106 .
- ↑ SR Lewin, C. Rouzioux: HIV cure and eradication: how will we get from the laboratory to effective clinical trials? . In: AIDS . 25, No. 7, April 2011, pp. 885-897. doi : 10.1097 / QAD.0b013e3283467041 .
- ^ H. Deng, R. Liu et al .: Identification of a major co-receptor for primary isolates of HIV-1. In: Nature , Volume 381, Number 6584, June 1996, pp. 661-666. doi: 10.1038 / 381661a0 . PMID 8649511 .
- ↑ YR Zou, AH Kottmann et al .: Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. In: Nature , Volume 393, Number 6685, June 1998, pp. 595-599, doi: 10.1038 / 31269 . PMID 9634238 .
- ↑ YR Zou et al .: Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. In: Nature , Vol. 393, No. 6685, 1998, pp. 595-599, PMID 9634238 .
- ↑ C. Winkler, W. Modi et al .: Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. ALIVE Study, Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC) In: Science , Volume 279, Number 5349, January 1998, pp. 389-393, PMID 9430590 .
- ↑ D. Schols, S. Struyf et al .: Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. In: The Journal of experimental medicine. Volume 186, Number 8, October 1997, pp. 1383-1388, ISSN 0022-1007 . PMID 9334378 . PMC 2199084 (free full text).
- ↑ T. Murakami, T. Nakajima et al .: A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. In: The Journal of experimental medicine , Volume 186, Number 8, October 1997, pp. 1389-1393, ISSN 0022-1007 . PMID 9334379 . PMC 2199089 (free full text).
- ↑ DM Knipe, Peter M. Howley , DE Griffin (Ed.): Fields Virology. 5th edition, Lippincott Williams & Wilkins, Philadelphia 2007, ISBN 978-0-7817-6060-7 .
- ↑ V. Poletti, F. Mavilio: Interactions between Retroviruses and the host cell genomes. In: Molecular therapy. Methods & clinical development. Volume 8, March 2018, pp. 31–41, doi: 10.1016 / j.omtm.2017.10.001 , PMID 29159201 , PMC 5684498 (free full text).
- ↑ F. Maldarelli, X. Wu, L. Su, FR Simonetti, W. Shao, S. Hill, J. Spindler, AL Ferris, Mellors JW, MF Kearney, Coffin JM, Hughes SH: HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. In: Science . Volume 345, number 6193, July 2014, pp. 179-183, doi: 10.1126 / science.1254194 , PMID 24968937 , PMC 4262401 (free full text).
- ^ G. Li, E. De Clercq: HIV Genome-Wide Protein Associations: a Review of 30 Years of Research. In: Microbiology and molecular biology reviews: MMBR. Volume 80, number 3, 2016-09, pp. 679-731, doi: 10.1128 / MMBR.00065-15 , PMID 27357278 , PMC 4981665 (free full text).
- ↑ a b C. Liu, JR Perilla, J. Ning, M. Lu, G. Hou, R. Ramalho, BA Himes, G. Zhao, GJ Bedwell, IJ Byeon, J. Ahn, AM Gronenborn, PE Prevelige, I Rousso, C. Aiken, T. Polenova, K. Schulten, P. Zhang: Cyclophilin A stabilizes the HIV-1 capsid through a novel non-canonical binding site. In: Nature Communications . Volume 7, 2016, p. 10714, doi: 10.1038 / ncomms10714 , PMID 26940118 .
- ↑ Armin Schafberger, Holger Sweers: HIV / AIDS - today's knowledge. German AIDS Aid e. V., 2008, p. 7 , accessed on January 15, 2016 .
- ^ ES Rosenberg, M. Altfeld u. a .: Immune control of HIV-1 after early treatment of acute infection. In: Nature. Volume 407, Number 6803, September 2000, pp. 523-526, ISSN 0028-0836 . doi: 10.1038 / 35035103 . PMID 11029005 .
- ↑ M. Altfeld, ES Rosenberg u. a .: Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. In: The Journal of experimental medicine. Volume 193, Number 2, January 2001, pp. 169-180, ISSN 0022-1007 . PMID 11148221 . PMC 2193337 (free full text).
- ^ HIV causes structural heart disease. (No longer available online.) In: escardio.org. December 11, 2013, archived from the original on December 27, 2014 ; accessed on December 27, 2014 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ L. Lopalco: Humoral immunity in HIV-1 exposure: cause or effect of HIV resistance? In: Current HIV research. Volume 2, Number 2, April 2004, pp. 127-139, ISSN 1570-162X . PMID 15078177 .
- ↑ F. Porichis, DE Kaufmann: HIV-specific CD4 T cells and immune control of viral replication. In: Current opinion in HIV and AIDS. Volume 6, Number 3, May 2011, pp. 174-180, ISSN 1746-6318 . doi: 10.1097 / COH.0b013e3283454058 . PMID 21502921 . PMC 3265969 (free full text).
- ↑ M. Dean, M. Carrington et al. a .: Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. In: Science. Volume 273, Number 5283, September 1996, pp. 1856-1862, ISSN 0036-8075 . PMID 8791590 .
- ^ R. Liu, WA Paxton et al. a .: Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. In: Cell. Volume 86, Number 3, August 1996, pp. 367-377, ISSN 0092-8674 . PMID 8756719 .
- ↑ A. Djordjevic, M. Veljkovic et al. a .: The presence of antibodies recognizing a peptide derived from the second conserved region of HIV-1 gp120 correlates with non-progressive HIV infection. In: Current HIV research. Volume 5, Number 5, September 2007, pp. 443-448, ISSN 1873-4251 . PMID 17896963 .
- ↑ HIV test & advice. (No longer available online.) Federal Center for Health Education (BZgA), archived from the original on July 15, 2014 ; Retrieved July 7, 2014 .
- ↑ The HIV test Infection risk - when to test? (PDF) LADR
- ^ Cohen et al .: The Detection of Acute HIV Infection . In: The Journal of Infectious Diseases . 202, 2010, pp. S270-S277. doi : 10.1086 / 655651 . PMID 20846033 .
- ↑ Diagnostic window: Shortened from twelve to six weeks from 2015! German AIDS Aid; accessed on January 15, 2016.
- ↑ Answers to frequently asked questions about HIV infection and AIDS . Robert Koch Institute , July 27, 2015; accessed on January 15, 2016.
- ↑ L. Simonsen, J. Buffington et al. a .: Multiple false reactions in viral antibody screening assays after influenza vaccination. In: American journal of epidemiology , Volume 141, Number 11, June 1995, pp. 1089-1096, ISSN 0002-9262 . PMID 7539579 .
- ↑ a b c Dustin Grunert: Overall fewer new infections . In: Deutsches Ärzteblatt , 2019, volume 116, issue 48, November 29, 2019, p. A2240.