Dependence on psychoactive substances
| Classification according to ICD-10 | |
|---|---|
| F10.2 | Mental and behavioral disorders caused by alcohol (addiction syndrome) |
| F11.2 | Mental and behavioral disorders caused by opioids (addiction syndrome) |
| F12.2 | Mental and behavioral disorders caused by cannabinoids (addiction syndrome) |
| F13.2 | Mental and behavioral disorders caused by sedatives or hypnotics (addiction syndrome) |
| F14.2 | Mental and behavioral disorders caused by cocaine (addiction syndrome) |
| F15.2 | Mental and behavioral disorders from other stimulants, including caffeine (addiction syndrome) |
| F16.2 | Mental and behavioral disorders caused by hallucinogens (addiction syndrome) |
| F17.2 | Mental and behavioral disorders caused by tobacco (addiction syndrome) |
| F18.2 | Mental and behavioral disorders due to volatile solvents (addiction syndrome) |
| F19.2 | Mental and behavioral disorders due to multiple substance use and consumption of other psychotropic substances (addiction syndrome) |
| ICD-10 online (WHO version 2019) | |

With dependence on psychoactive substances is referred to a group of health problems due to repeated ingestion of various psychotropic substances . A strong, periodic or permanent desire for substance is considered typical . It can lead to neglect of other obligations or activities - possibly even progressive. A possible loss of control with compulsive substance use cannot be ruled out.
Depending on the substance, there may be an increase in tolerance and an increase in dose and - if not ingested - withdrawal symptoms may occur. If psychoactive substances are taken within a social context , the dependence must be viewed in the context of complex interactions between social and biological processes.
Medical definition
According to the definitions of the World Health Organization (WHO) - see box above right: Classification according to ICD-10 - addiction typically consists of a strong desire to ingest a substance, difficulties in controlling its use, and continued use despite harmful consequences.
frequency
For the frequency of the individual addiction syndromes, see the respective main article on psychotropic substances.
On the basis of scientific studies, the number of people manifestly dependent on medication in Germany is estimated at around 1.4–1.9 million. In about 80% of the cases, this is a dependence on benzodiazepines , which have a high potential for dependence.
Dependency potential
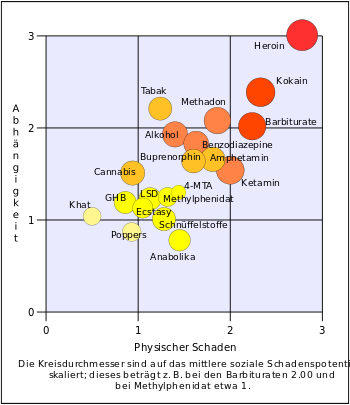
Psychotropic substances , i.e. substances that influence thinking and feelings, can, with different potentials, induce a person to unconditionally re- use , i.e. lead to dependence. This property is called dependency potential or addictive potential .
According to a theory by Hollister (1978), the potential for dependence on substances with a very short or very long half-life should be lower. It is assumed that substances with a rapid influx of substances have the highest potential for dependence.
2007 study
According to a British study from 2007, the substances with the highest addiction potential (in descending order according to addiction potential, scale values in brackets after) are:
- The opioid heroin (3.00),
- the stimulant cocaine (2.39),
- Tobacco (2.21),
- the opioid methadone (2.08),
- and sleeping pills from the group of barbiturates (2.01).
Substances with medium dependency potential:
- Alcohol (1.93),
- Benzodiazepines ( anti-anxiety substances , sleeping pills ) (1.83),
- the stimulant amphetamine (1.67),
- the opioid buprenorphine (1.64),
- Ketamine (1.54),
- and cannabis (see also: marijuana , hashish , hashish oil ) (1.51).
Substances with low addiction potential:
- 4-MTA (ecstasy-like, drug scene name Flatliners) (1.30),
- Methylphenidate (known as Ritalin) (1.25),
- LSD (1.23),
- GHB (1.19),
- Ecstasy (1.13),
- Khat (1.04),
- and inhalants (1.01).
Substances without pronounced dependence potential:
- anabolic steroids (anabolic steroids) (0.88),
- and poppers (0.87).
Study from 2017
In 2017, the World Commission on Drug Policy published a partly different order .
11.6% of those who use drugs become addicted on average:
- 32% for tobacco
- 23% for heroin
- 17% for cocaine
- 15% for alcohol
- 9% for cannabis.
More about individual substances
- alcohol
- Although alcoholic beverages are legal in many countries, ethanol has a dependency potential comparable to opiates, barbiturates and cocaine. Alcohol dependence develops after long-term increased alcohol consumption and leads to alcohol sickness . Delirium tremens can occur when the substance is discontinued . The number of alcohol addicts is high because alcohol is a cheap and easily obtainable drug that is also socially recognized, e.g. B. the consumption at social occasions.
- Amphetamines (speed)
- Amphetamines can trigger a strong psychological dependency, as the effect promises increased performance, improved concentration and euphoria as a party drug. The risk of addiction depends on the genetic disposition and the psychological situation of the person. In animal experiments, some individuals were able to regulate their consumption for the rest of their lives, while 50% developed psychological dependency after some time with strong tolerance development.
- cannabis
- The likelihood of becoming dependent on cannabis use is increased by a number of psychosocial factors. The development of an addiction syndrome has been assigned to an experimentally observed underfunction of the reward system in the human brain.
- Hallucinogens
- The hallucinogens are in different categories divided . Psychedelics such as LSD , mescaline and hallucinogenic mushrooms usually do not cause addiction. Hallucinogens from the group of dissociatives , such as ketamine , on the other hand, cause addiction.
- Heroin and other opiates / opioids
- Heroin is one of the substances with the highest potential for addiction. Due to the euphoric effect, a psychological dependency quickly develops, as the consumer forgets negative thoughts and worries while intoxicated. The risk of addiction is therefore determined according to the psychosocial situation of the consumer. After frequent use, opiates become dependent, with withdrawal being experienced as extremely unpleasant and therefore offering the person affected a (negative) motivation to continue consuming in order to avoid or reduce the withdrawal symptoms.
- Cocaine and crack
- The potential for addiction to cocaine is also considered to be very high. Until 1980 the prevailing view was that cocaine did not cause addiction in the sense of withdrawal symptoms. A few years later this view turned into its opposite, among other things after corresponding changes in the brain became known. Mental withdrawal syndromes due to cocaine use have been scientifically established since the 1990s and are increasingly being researched on a biochemical level. The drug crack , made from cocaine , also causes addiction.
- Medication
- About 4 to 5% of drugs have a potential for abuse or dependence. This applies in particular to hypnotics , sedatives and tranquillants, as well as psychostimulants , and also to opioid-containing analgesics . Patients usually expect a positive effect from drugs, so it may be difficult to correctly assess the potential risk of abuse or addiction when taking them.
- MDMA (ecstasy)
- The addiction rate of common MDMA users according to the criteria of DSM IV was estimated in a systematic review from 2011 to be around 15%. On the other hand, long-term damage (longer than 2 years) to nerve endings that work with dopamine or serotonin is also a general phenomenon in humans, which affects all consumers.
- Tobacco smoke
- For the dependence of tobacco smoke is nicotine responsible. Comparisons of animal studies and studies of human drug use show that pure nicotine has little potential for addiction, but tobacco cigarette smoke has a very high potential for addiction. In combination with other substances in tobacco smoke, nicotine has an extremely high potential for dependence and can very quickly lead to dependent behavior . According to a paper published in 2007 by D. Nutt et al. a. the addiction potential of tobacco smoke lies somewhere between alcohol and cocaine. More precisely, the physical addiction potential is that of alcohol or barbiturates and the psychological addiction potential that of cocaine. A comparison with addiction to opiates such as heroin is not indicated because this is much more difficult to treat and the withdrawal symptoms are more severe. A few cigarettes or a few days with little cigarette consumption are enough to become dependent. The potential for dependence on orally ingested nicotine is significantly lower, and patches have almost no potential for dependence.
- Above all, it is important that nicotine, in conjunction with other substances in tobacco smoke, subliminally creates the desire for a tobacco product and that the increasingly shorter habit-related stimulus-response interval creates an increasingly pronounced dependency in the form of increased tobacco consumption.
- Today we know that after three weeks of abstinence there is no longer any measurable change in the acetylcholine receptors - that is, they have returned to normal. During this time there can be restlessness and irritability up to aggressiveness and depression. At this point in time, the nicotine itself is no longer detectable in the brain (up to a maximum of three days after the end of nicotine consumption).
- As a result, one can establish a potential for dependency, which is rather unconscious, i.e. H. in unreflected everyday life, finds its greatest expression. In withdrawal phases it becomes apparent that these unconscious parts are now consciously processed by the withdrawing person.
- As a result, it can be stated that during withdrawal the dependence on the effects produced by the tobacco smoke is less important, as shown by many failed therapies with nicotine substitutes, but rather the learning process induced by the nicotinic stimulation of the nucleus accumbens . In a suitable way, this learning process can only be influenced or reversed by strong self-motivation or professional behavioral therapies. Nicotine replacement or medication can help with withdrawal.
- The likelihood of relapse among smokers who quit tobacco without aids is 97% within six months of quitting. Until 2012, it was assumed that nicotine replacement preparations with the correct dosage and further professional guidance could increase the chances of success by 3%. A recent study from 2012 found that relapse rates among those who used nicotine replacement supplements to quit were just as high as those who quit without aids.
- The psychological dependence due to imprinted behavior patterns that develop in the course of a “smoking career” can still be present years after withdrawal.
Multiple substance use
According to ICD-10 (F19.-), there is a disorder due to multiple substance use if the substance intake is chaotic and indiscriminate, or if components of different substances are inseparably mixed.
Theories on the development of addiction syndrome
Dependency is the result of a multi-causal process in which biological and social factors interact.
Genetic disposition
The likelihood of an addiction disease is also influenced by certain variants in the genetic make-up. However, to this day (2018) there is still a large gap between the very small individual effects of genetic deviations in this regard and the known extent of actual hereditary influence. Nevertheless, there are already very concrete results in the areas of nicotine, opioids , alcohol, cannabis and cocaine. Investigation methods in this area are family studies, adoption studies, twin studies , candidate gene discovery, genome-wide association studies and the analysis of copy number variants (CNV) in certain chromosome sections.
Neurobiological mechanisms of action

The emergence of a dependency is seen neurobiological an abnormal ( pathological ) form of an actually useful, biological learning process, the sensitization is called. A psychoactive substance causes changes on the surfaces and inside of nerve cells that increase the future desire to use this substance again. This sensitization of desire usually lasts long after withdrawal and therefore increases the risk of relapse. In contrast to the desire, the desired feeling ( euphoria ) is not increased, but rather weakens (development of tolerance ).
At the cellular and molecular level, sensitization is caused by changes in neurochemistry , neurophysiology , neuroanatomy, and gene expression . A single use of a substance can lead to long-lasting changes in the signal transmission of nerve cells. The most far-reaching changes happen during the developmental phases of the brain, most extreme during prenatal ( prenatal ) development.
In two reviews from 2016, dependence is described as a connection between three disease complexes, each of which is assigned to certain changes in certain networks of the brain. These are the complexes (1) pathological desire (addiction), (2) pathological withdrawal symptoms and (3) pathological expectations. In a subsequent review from 2018 it was described that this model - with small deviations - is also applicable to the abuse of cannabis .
Cross-sensitization of substances
Many studies have shown that repeated use of a substance can not only increase sensitivity to this substance ( sensitization ), but also sensitivity to other psychoactive substances. This process is known as cross-sensitization .
Compared to clinical studies , it is relatively easy to determine in animal experiments whether the consumption of one drug increases the later attractiveness of another drug. For example, cannabis use in animals increased self-administration of heroin, morphine and nicotine in follow-up experiments. Direct evidence has also been found that the mechanism of imprinting is an ongoing change in the brain's reward system. The importance of these results for the reward system in the human brain in relation to susceptibility to other drugs has been highlighted in several reviews .
Epidemiological Results
Denise Kandel , professor of sociomedical sciences in psychiatry at Columbia University and director of the epidemiology of substance abuse at the New York State Psychiatric Institute, and colleagues have published the results of several studies on the timing of initial drug use since 1975. It has been observed that the order of initial use of various drugs is not random, but rather shows trends. The established technique of longitudinal studies made it possible to precisely describe these trends by specifying probabilities .
A meta-analysis from 2018 came to the conclusion that the use of e -cigarettes significantly increases the likelihood of later use of conventional tobacco cigarettes:
"There is strong empirical evidence [emphasis in original] that e-cigarette use increases the risk of adolescents and young adults ever using flammable tobacco cigarettes."
Among other things, the authors put this into connection with the fact that the well-known sensitization of the brain by the nicotine of e-cigarettes - especially in adolescents - leads to an increased probability of further nicotine consumption.
Psychological conditions
Willful control of substance desires
Dependency includes impairment of perception, risk assessment and control of one's own behavior. The likelihood of automatic behavior related to substance use is increased. The possibility to choose between different decisions is thus often difficult, but by no means completely lost.
Concomitant illnesses and consequences
In addition to the addiction syndrome, there are a number of comorbidities .
Psychological comorbidities
Mental disorders can precede a dependency syndrome and are to be treated as concomitant diseases. Possible concomitant diseases can be B. anxiety disorders , depression , ADHD , adjustment disorders , personality disorders or psychoses . The mental disorders mentioned can also be the result of an addiction syndrome; in the case of psychosis, the term drug psychosis is common. Also, social isolation may be preceded by a dependency syndrome or consequence.
Consequences of the addiction syndrome
The consequences of the addiction syndrome depend largely on the psychotropic substance to which the addiction exists. See therefore the main article on the respective substance.
Orientation towards addiction is becoming more and more important in the life of those affected. The consumer's attention is increasingly shifted to the consumption and procurement of the psychoactive substance and the subsequent lingering in a state of intoxication. Other activities, interests and obligations are often neglected. Personality, or personal development, can be significantly impaired. In addition, there is a risk of procurement crime .
Prevention and therapy
Prevention
Newer approaches to drug prevention rely less on deterrence; H. the emphasis on often organism damaging properties, but increasingly on education. Deterrence was often not taken seriously by the target groups. Education is used as an alternative to repression-oriented drug policies, in which drug use is viewed as intolerable.
The aim of this prevention concept is to impart knowledge about the effects of a substance. This concerns both the expected pleasant effects or undesirable side effects, as well as the possible social and health damage. This knowledge should then enable you to make your own decision. Since it is not assumed that this decision will always be against consumption, the aim is to encourage safe handling, such as avoiding mixed consumption. The aim of prevention should be to work towards strengthening the personality and showing alternatives to substance use. Potential users must be able to experience these alternatives - for example, the experience that after two hours of “exhausting” one feels at least as “relaxed” as when consuming cannabis.
Prevention and substance-specific information should begin as early as possible, as the first contact with drugs often takes place in adolescence .
therapy
A drug treatment for a dependency syndrome has permanent waiver ( abstinence ) to the dependence-producing substance to the target and may include the following:
- Withdrawal from the addictive substance
- Substitution by a substance with little or no harmful effect
- Psychotherapeutic treatment (short-term interventions, especially long-term weaning) in a specialist clinic , whereby these focus on possible deficits in the personality development of the patient and are strongly oriented towards individual possibilities
- physical therapy
- Participation in self-help group
- Treatment of relatives / caregivers; see also: codependency
In the treatment of opiate addicts, the (sometimes permanent) administration of a substitute as part of a substitution therapy can lead to a significant harm reduction . The requirement for abstinence as the sole treatment goal is abandoned or decided in each individual case (and possibly again and again). The effectiveness of permanent substitution has been convincingly proven and is also recognized by the legislature. Drugs are also being developed that are not to be regarded as substitutions, but are intended to specifically combat the symptoms of dependence, e.g. B. Clofenciclan .
The treatment of addictions has developed into a specialty of medicine, which today is increasingly based on the knowledge of neurobiology and can offer a whole spectrum of therapeutic methods. Educational measures can also be part of a therapy, especially for young people.
Outdated ideas
The term “psychological dependence” originates from the observation that dependence syndrome is sometimes primarily experienced through negative feelings ( emotions ) such as depression or anxiety . In this context, one spoke of “physical dependency” when additional vegetative disorders such as restlessness or circulatory disorders occurred . However, the boundaries between the two terms were always fluid, as emotional and vegetative effects always influence each other.
Since the increasing description of changes in the brain in connection with addiction syndromes, the differences between so-called psychological and physical effects have become superfluous since the 1990s . The observed changes in the brain affect both types of effects equally. In science, the distinction has since almost died out. The last PubMed- listed review article by western authors with "psychological dependence" in the title appeared in 1990.
Delimitations
Other forms of addiction that are not characterized by the use of psychotropic substances are summarized in the article Addiction (Medicine) . If the use of psychoactive substances leads to a violation of the law, dependence or substance use is defined as criminal. Questions of this kind then usually touch the fields of toxicology in forensic medicine .
See also
- Addiction medicine
- Gateway drug
- Weaning: Tapering · End point method
- Special substances: opiate addiction · Tobacco additive · Harmful use of benzodiazepines
- Substance-free addiction
- Addicted - Protocol of a helplessness (long-term documentary)
AWMF guidelines
Guidelines of the Working Group of Scientific Medical Societies :
- S3 guideline: Metamphetamine-related disorders (as of 2016)
- S3 guideline: "Screening, diagnosis and treatment of alcohol-related disorders" (as of 2015)
- S3 guideline: "Screening, diagnosis and treatment of harmful and dependent tobacco consumption" (status: 2015)
- S2 guideline: Mental and behavioral disorders caused by cocaine, amphetamines, ecstasy and hallucinogens , AWMF register number 076/007 (online: full text ( Memento from January 2, 2005 in the Internet Archive )), status 10/2004
- Drug addiction. ( Memento from February 4, 2010 in the Internet Archive ) Uni Düsseldorf
literature
General
- Günter Amendt : No drugs - No future. Drugs in the age of globalization . Europa-Verlag, Hamburg, Vienna 2003, ISBN 3-203-75013-9 .
- Klaus Behrendt, Markus Backmund, Jens Reimer (responsible for the content): drug addiction (= addiction medicine series . Volume 4 ). 4th edition. German Center for Addiction Issues e. V., Hamm 2016, ISBN 978-3-937587-03-5 ( full text [PDF; 2,3 MB ]).
- Stefan Böhm: Addictive substances . In: Michael Freissmuth u. a .: Pharmacology and toxicology: From the molecular basis to pharmacotherapy . Springer Berlin, 2016, ISBN 978-3-662-46689-6 . , Pp. 327–336 ( preview Google Books ).
- Andreas Heinz , Anil Batra , Norbert Scherbaum, Euphrosyne Gouzoulis-Mayfrank : Neurobiology of Dependence. Basics and consequences for diagnosis and therapy of addictive diseases . Kohlhammer, Stuttgart 2012, ISBN 978-3-17-021474-3 .
- Steven B. Karch (Ed.): Addiction and the Medical Complications of Drug Abuse , CRC Press, London 2008, ISBN 978-1-4200-5444-6 ( preview Google Books ).
- George F. Koob, Michael A. Arends, Michel Le Moal: Drugs, addiction, and the brain. Academic Press, Oxford 2014, ISBN 978-0-12-386959-3 ( preview Google Books ).
- Victor R. Preedy: Neuropathology of Drug Addictions and Substance Misuse: Volume 1. Foundations of Understanding, Tobacco, Alcohol, Cannabinoids and Opioids , Academic Press, London 2016, ISBN 978-0-12-800376-3 ( preview Google Books ).
- Michael Soyka u. a .: Addiction medicine . Elsevier, Munich, 2019, ISBN 978-3-437-17051-5 . ( Preview Google Books ).
- Felix Tretter (Hrsg.): Addiction medicine compact: Addiction diseases in clinic and practice - ready to hand . 3rd, updated and expanded edition. Schattauer Verlag, Stuttgart 2017, ISBN 978-3-7945-3162-2 ( preview Google Books ).
- Jens Ullrich: Addiction, addiction and harmful use , in: Maximilian von Heyden, Henrik Jungaberle, Tomislav Majić (eds.): Handbook Psychoactive Substances , Springer Berlin, 2018, ISBN 978-3-642-55125-3 , pp. 207– 215 ( preview Google Books ).
counselor
- Karin Elsesser, Gudrun Sartory: Counselor drug addiction. Information for those affected and their relatives . Hogrefe Verlag, Göttingen 2005, ISBN 978-3-8444-1767-8 ( preview Google Books ).
- Karl-Ludwig Täschner, Benedikt Bloching, Gerhard Bühringer, Gerhard A. Wiesbeck: Therapy of drug addiction . 2nd, completely revised and expanded edition. Kohlhammer Verlag, Stuttgart 2010, ISBN 978-3-17-026593-6 , ( preview Google Books ).
- Maree Teesson, Louisa Degenhardt, Wayne Hall: Addictive substances and addiction: forms - effects - interventions . Huber Verlag, Bern 2008, ISBN 978-3-456-84476-3 .
history
- Claudia Wiesemann : The secret illness. A history of the term addiction. (= Medicine and Philosophy. Volume 4). Frommann-Holzboog, Stuttgart 2000, ISBN 3-7728-2000-X .
- J. Olds, P. Milner: Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. In: Journal of comparative and physiological psychology. Volume 47, Number 6, December 1954, pp. 419-427, PMID 13233369 .
Web links
- Entry of dependence, physical and psychological in the Lexicon of Psychology on Spektrum.de (WHO terminology)
- What is addiction / an addiction disease? - Neurologists and psychiatrists online (terminology and statistics)
- Volkart Wildermuth : The dependent brain. New perspectives for the therapy of addictions. In: Wissenschaft im Brennpunkt , Deutschlandfunk, March 5, 2006
Individual evidence
- ↑ a b Drug addiction. Website of the German Medical Association ( Memento from January 16, 2013 in the Internet Archive )
- ↑ a b c d e David Nutt, Leslie A King, William Saulsbury, Colin Blakemore: Development of a rational scale to assess the harm of drugs of potential misuse . In: The Lancet . tape 369 , March 2007, p. 1047-1053 , doi : 10.1016 / S0140-6736 (07) 60464-4 .
- ^ Robert Gable: Drug Toxicity. Retrieved February 17, 2011 .
- ↑ RS Gable: Acute toxicity of drugs versus regulatory status . In JM Fish (Ed.): Drugs and Society: US Public Policy. Rowman & Littlefield Publishers, Lanham MD 2005, ISBN 0-7425-4244-0 , pp. 149-162.
- ^ Lutz G. Schmidt, Markus Gastpar, Peter Falkai: Evidence-based addiction medicine: treatment guidelines for substance-related disorders. Deutscher Ärzte-Verlag, 2006, ISBN 3-7691-0520-6 , p. 276.
- ↑ Mean dependency potential ≥ 2.00 in the scale of the study by Nutt u. a.
- ↑ Mean dependency potentials ≥ 1.50 and <2.00 in the scale of the study by Nutt u. a.
- ↑ Mean value of dependency potentials ≥ 1.00 and <1.50 in the scale of the study by Nutt u. a.
- ↑ Mean dependency potential <1.00 in the scale of the study by Nutt u. a.
- ↑ Germany in Rush - The Lost Battle Against Drugs Film by Anna Feist, ZDFzoom from October 23, 2019, available in the media library until October 23, 2020, the numbers appear at the end
- ↑ Alcohol - the intoxicating luxury or addictive substance ( Memento from December 3, 2013 in the Internet Archive )
- ↑ Gabriel Galli, Jochen Wolffgramm: Long-term voluntary D-amphetamine consumption and behavioral predictors for subsequent D-amphetamine addiction in rats. In: Science. 73/1, 2004, pp. 51-60. PMID 14687959
- ^ S. Schlossarek, J. Kempkensteffen, J. Reimer, U. Verthein: Psychosocial Determinants of Cannabis Dependence: A Systematic Review of the Literature. In: European addiction research. Volume 22, number 3, 2016, pp. 131–144, doi: 10.1159 / 000441777 , PMID 26551358 (free full text) (review).
- ^ ZD Cooper, M. Haney: Actions of delta-9-tetrahydrocannabinol in cannabis: relation to use, abuse, dependence. In: International Review of Psychiatry. Volume 21, number 2, April 2009, pp. 104-112, doi: 10.1080 / 09540260902782752 , PMID 19367504 , PMC 2731700 (free full text) (review).
- Jump up ↑ E. van de Giessen, JJ Weinstein, CM Cassidy, M. Haney, Z. Dong, R. Ghazzaoui, N. Ojeil, LS Kegeles, X. Xu, NP Vadhan, ND Volkow, M. Slifstein, A. Abi- Dargham: Deficits in striatal dopamine release in cannabis dependence. In: Molecular Psychiatry. Volume 22, number 1, 01 2017, pp. 68-75, doi: 10.1038 / mp.2016.21 , PMID 27001613 , PMC 5033654 (free full text).
- ^ MA Bloomfield, AH Ashok, ND Volkow, OD Howes: The effects of Δ-tetrahydrocannabinol on the dopamine system. In: Nature . Volume 539, number 7629, 11 2016, pp. 369–377, doi: 10.1038 / nature20153 , PMID 27853201 , PMC 5123717 (free full text) (review).
- ↑ a b c E. R. Korpi, B. den Hollander, U. Farooq, E. Vashchinkina, R. Rajkumar, DJ Nutt, P. Hyytiä, GS Dawe: Mechanisms of Action and Persistent Neuroplasticity by Drugs of Abuse. In: Pharmacological Reviews . Volume 67, number 4, October 2015, pp. 872-1004, doi: 10.1124 / pr.115.010967 , PMID 26403687 (free full text) (review).
- ↑ D. De Gregorio, S. Comai, L. Posa, G. Gobbi: d-Lysergic Acid Diethylamide (LSD) as a Model of Psychosis: Mechanism of Action and Pharmacology. In: International Journal of Molecular Sciences . Volume 17, number 11, November 2016, p., Doi: 10.3390 / ijms17111953 , PMID 27886063 , PMC 5133947 (free full text) (review).
- ^ CJ Morgan, HV Curran: Ketamine use: a review. In: Addiction. Volume 107, number 1, January 2012, pp. 27–38, doi: 10.1111 / j.1360-0443.2011.03576.x , PMID 21777321 (review), reseaualto.be (PDF)
- ↑ K. Xu, RH Lipsky: Repeated ketamine administration alters N-methyl-D-aspartic acid receptor subunit gene expression: implication of genetic vulnerability for ketamine abuse and ketamine psychosis in humans. In: Experimental biology and medicine. Volume 240, number 2, February 2015, pp. 145-155, doi: 10.1177 / 1535370214549531 , PMID 25245072 , PMC 4469194 (free full text) (review).
- ^ FH Gawin, HD Kleber: Evolving conceptualizations of cocaine dependence. In: The Yale journal of biology and medicine. Volume 61, Number 2, 1988 Mar-Apr, pp 123-136, PMID 3043925 , PMC 2590292 (free full text) (review).
- ↑ NS Pilotte, LG Sharpe: Cocaine withdrawal of old regulatory elements of dopamine neurons. In: NIDA research monograph. Volume 163, 1996, pp. 193-202, PMID 8809860 (Review), drugabuse.gov (PDF)
- ↑ MJ Kuhar, NS Pilotte: Neurochemical changes in cocaine withdrawal. In: Trends in pharmacological sciences. Volume 17, Number 7, July 1996, pp. 260-264, PMID 8756185 (review).
- ↑ NS Pilotte: Neurochemistry of cocaine withdrawal. In: Current opinion in neurology. Volume 10, Number 6, December 1997, pp. 534-538, PMID 9425570 (review).
- ^ XT Hu: Cocaine withdrawal and neuro-adaptations in ion channel function. In: Molecular neurobiology. Volume 35, Number 1, February 2007, pp. 95-112, PMID 17519508 (review).
- ↑ a b J. Cabana-Domínguez, C. Roncero, L. Grau-López, L. Rodríguez-Cintas, C. Barral, AC Abad, G. Erikson, NE Wineinger, B. Torrico, C. Arenas, M. Casas , M. Ribasés, B. Cormand, N. Fernàndez-Castillo: A Highly Polymorphic Copy Number Variant in the NSF Gene is Associated with Cocaine Dependence. In: Scientific Reports . Volume 6, 08 2016, p. 31033, doi: 10.1038 / srep31033 , PMID 27498889 , PMC 4976312 (free full text).
- ^ GT McClelland: The effects and management of crack cocaine dependence. In: Nursing times. Volume 101, Number 29, 2005 Jul 19-25, pp. 26-27, PMID 16052938 (Review)
- ↑ T. Steinkellner, M. Freissmuth, HH Sitte, T. Montgomery: The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, 'Ecstasy'), methamphetamine and D-amphetamine. In: Biological chemistry. Volume 392, number 1–2, January 2011, pp. 103–115, doi: 10.1515 / BC.2011.016 , PMID 21194370 , PMC 4497800 (free full text) (review).
- ↑ LE Halpin, SA Collins, BK Yamamoto: Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. In: Life sciences. Volume 97, number 1, February 2014, pp. 37-44, doi: 10.1016 / j.lfs.2013.07.014 , PMID 23892199 , PMC 3870191 (free full text) (review).
- ↑ Determinants of Tobacco Use and Renaming the FTND to the Fagerström Test for Cigarette Dependence . In: ntr.oxfordjournals.org , accessed July 28, 2013.
- ↑ James D Belluzzi et al. a .: Monoamine Oxidase Inhibitors Allow Locomotor and Rewarding Responses to Nicotine . In: Nature . December 14, 2005, accessed August 1, 2013.
- ↑ James D Belluzzi et al. a .: Acetaldehyde Enhances Acquisition of Nicotine Self-Administration in Adolescent Rats . In: Nature. October 20, 2004, accessed August 1, 2013.
- ^ JE Rose, WA Corrigall: Nicotine self-administration in animals and humans: similarities and differences . March 1997. PMID 9089846 .
- ↑ Questions about tobacco additives: Is the development of nicotine addiction dose-dependent? 2010. SCENIHR. Retrieved July 29, 2013.
- ↑ How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General, Nicotine Addiction: Past and Present . Surgeon General (US), 2010, accessed July 29, 2013.
- ↑ Development of a rational scale to assess the harm of drugs of potential misuse. (PDF; 127 kB) In: dobrochan.ru. 2007, accessed March 9, 2013 .
- ↑ Harm reduction on nicotine addiction . (PDF) In: rcplondon.ac.uk. P. 98/99
- ↑ LF Stead u. a .: Nicotine replacement therapy for smoking cessation . Cochrane Tobacco Addiction Group, July 16, 2008, doi: 10.1002 / 14651858.CD000146.pub3
- ↑ Nicotine replacement and other smoking cessation drugs . In: DKFZ.de , accessed on March 6, 2013.
- ↑ HR Alpert, GN Connolly, L. Biener: A prospective cohort study challenging the effectiveness of population-based medical intervention for smoking cessation. In: Tobacco Control . Volume 22, number 1, January 2013, pp. 32-37, doi: 10.1136 / tobaccocontrol-2011-050129 . PMID 22234781 , ISSN 1468-3318 .
- ↑ ML Copersino: Cognitive Mechanisms and Therapeutic Targets of Addiction. In: Current opinion in behavioral sciences. Volume 13, February 2017, pp. 91-98, doi: 10.1016 / j.cobeha.2016.11.005 , PMID 28603756 , PMC 5461927 (free full text) (review).
- ^ S. Wang, Z. Yang, JZ Ma, TJ Payne, MD Li: Introduction to deep sequencing and its application to drug addiction research with a focus on rare variants. In: Molecular neurobiology. Volume 49, number 1, February 2014, pp. 601-614, doi: 10.1007 / s12035-013-8541-4 , PMID 23990377 , PMC 3948219 (free full text) (review).
- ↑ FM Vassoler, G. Sadri-Vakili: Mechanisms of transgenerational inheritance of addictive-like behaviors. In: Neuroscience. Volume 264, April 2014, pp. 198-206, doi: 10.1016 / j.neuroscience.2013.07.064 , PMID 23920159 , PMC 3872494 (free full text) (review).
- ↑ BM Sharp, H. Chen: Neurogenetic determinants and mechanisms of addiction to nicotine and smoked tobacco. In: The European journal of neuroscience. [electronic publication before printing] September 2018, doi: 10.1111 / ejn.14171 , PMID 30256469 (review), wiley.com (PDF)
- ↑ P. Gorwood, Y. Le Strat, N. Ramoz: Genetics of addictive behavior: the example of nicotine dependence. In: Dialogues in clinical neuroscience. Volume 19, number 3, September 2017, pp. 237–245, PMID 29302221 , PMC 5741107 (free full text) (review).
- ^ RC Crist, BC Reiner, WH Berrettini: A review of opioid addiction genetics. In: Current opinion in psychology. [electronic publication before printing] August 2018, doi: 10.1016 / j.copsyc.2018.07.014 , PMID 30118972 (review).
- ↑ I. Prytkova, A. Goate, RP Hart, PA Slesinger: Genetics of Alcohol Use Disorders: A Role for Induced Pluripotent Stem Cells? In: Alcoholism, clinical and experimental research. Volume 42, number 9, September 2018, pp. 1572–1590, doi: 10.1111 / acer.13811 , PMID 29897633 , PMC 6120805 (free full text) (review).
- ↑ MT Reilly, A. Noronha, D. Goldman, GF Koob: Genetic studies of alcohol dependence in the context of the addiction cycle. In: Neuropharmacology. Volume 122, August 2017, pp. 3-21, doi: 10.1016 / j.neuropharm.2017.01.017 , PMID 28118990 (review).
- ^ AB Hart, HR Kranzler: Alcohol Dependence Genetics: Lessons Learned From Genome-Wide Association Studies (GWAS) and Post-GWAS Analyzes. In: Alcoholism, clinical and experimental research. Volume 39, number 8, August 2015, pp. 1312-1327, doi: 10.1111 / acer.12792 , PMID 26110981 , PMC 4515198 (free full text) (review).
- ↑ KJ Verweij, BP Zietsch, MT Lynskey, SE Medland, MC Neale, NG Martin, DI Boomsma, JM Vink: Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. In: Addiction. Volume 105, number 3, March 2010, pp. 417-430, doi: 10.1111 / j.1360-0443.2009.02831.x , PMID 20402985 , PMC 2858354 (free full text) (review).
- ^ A. Agrawal, MT Lynskey: Candidate genes for cannabis use disorders: findings, challenges and directions. In: Addiction. Volume 104, number 4, April 2009, pp. 518-532, doi: 10.1111 / j.1360-0443.2009.02504.x , PMID 19335651 , PMC 2703791 (free full text) (review).
- ↑ JD Steketee, PW Kalivas: Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. In: Pharmacological reviews. Volume 63, number 2, June 2011, pp. 348-365, doi: 10.1124 / pr.109.001933 , PMID 21490129 , PMC 3082449 (free full text) (review).
- ^ MJ Robinson, AM Fischer, A. Ahuja, EN Lesser, H. Maniates: Roles of "Wanting" and "Liking" in Motivating Behavior: Gambling, Food, and Drug Addictions. In: Current topics in behavioral neurosciences. Volume 27, 2016, pp. 105-136, doi : 10.1007 / 7854_2015_387 , PMID 26407959 (Review), wesleyan.edu (PDF)
- ^ CM Olsen: Natural rewards, neuroplasticity, and non-drug addictions. In: Neuropharmacology. Volume 61, number 7, December 2011, pp. 1109-1122, doi: 10.1016 / j.neuropharm.2011.03.010 , PMID 21459101 , PMC 3139704 (free full text) (review).
- ↑ ND Volkow , M. Morales: The Brain on Drugs: From Reward to Addiction. In: Cell . Volume 162, number 4, August 2015, pp. 712-725, doi: 10.1016 / j.cell.2015.07.046 , PMID 26276628 (free full text) (review).
- ^ EJ Nestler: Epigenetic mechanisms of drug addiction. In: Neuropharmacology. Volume 76 Pt B, January 2014, pp. 259-268, doi: 10.1016 / j.neuropharm.2013.04.004 , PMID 23643695 , PMC 3766384 (free full text) (review).
- ↑ DM Walker, EJ Nestler: Neuroepigenetics and addiction. In: Handbook of clinical neurology. Volume 148, 2018, pp. 747-765, doi: 10.1016 / B978-0-444-64076-5.00048-X , PMID 29478612 , PMC 5868351 (free full text) (review).
- ^ GF Koob, ND Volkow: Neurobiology of addiction: a neurocircuitry analysis. In: The lancet. Psychiatry. Volume 3, number 8, August 2016, pp. 760-773, doi: 10.1016 / S2215-0366 (16) 00104-8 , PMID 27475769 , PMC 6135092 (free full text) (review).
- ↑ ND Volkow, GF Koob, AT McLellan: Neurobiologic Advances from the Brain Disease Model of Addiction. In: The New England Journal of Medicine . Volume 374, number 4, January 2016, pp. 363-371, doi: 10.1056 / NEJMra1511480 , PMID 26816013 , PMC 6135257 (free full text) (review).
- ↑ A. Zehra, J. Burns, CK Liu, P. Manza, CE Wiers, ND Volkow , GJ Wang: Cannabis Addiction and the Brain: a Review. In: Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. Volume 13, Number 4, December 2018, pp. 438–452, doi: 10.1007 / s11481-018-9782-9 , PMID 29556883 (Review), springer.com (PDF)
- ↑ Peter Miller (Ed.): Biological research on addiction . Elsevier, Amsterdam, Boston, 2013, ISBN 978-0-12-398360-2 , p. 171, OCLC 828298745 . Preview of Google Books .
- ↑ a b H. C. Tomasiewicz, MM Jacobs, MB Wilkinson, SP Wilson, EJ Nestler, YL Hurd: proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. In: Biological psychiatry. Volume 72, number 10, November 2012, pp. 803-810, doi : 10.1016 / j.biopsych.2012.04.026 , PMID 22683090 , PMC 3440551 (free full text).
- ↑ a b M. Ellgren, SM Spano, YL Hurd: Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. In: Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. Volume 32, Number 3, March 2007, pp. 607-615, doi: 10.1038 / sj.npp.1301127 , PMID 16823391 .
- ↑ a b L. H. Parsons, YL Hurd: Endocannabinoid signaling in reward and addiction. In: Nature reviews. Neuroscience. Volume 16, number 10, October 2015, pp. 579-594, doi: 10.1038 / nrn4004 , PMID 26373473 , PMC 4652927 (free full text) (review).
- ↑ C. Cadoni, A. Pisanu, M. Solinas, E. Acquas, G. Di Chiara: Behavioral sensitization after repeated exposure to Delta 9-tetrahydrocannabinol and cross-sensitization with morphine. In: Psychopharmacology. Volume 158, Number 3, November 2001, pp. 259-266, doi: 10.1007 / s002130100875 , PMID 11713615 .
- ^ WL Sun, PM Quizon, J. Zhu: Molecular Mechanism: ERK Signaling, Drug Addiction, and Behavioral Effects. In: Progress in molecular biology and translational science. Volume 137, 2016, pp. 1-40, doi: 10.1016 / bs.pmbts.2015.10.017 , PMID 26809997 , PMC 5330621 (free full text) (review).
- ↑ LV Panlilio, C. Zanettini, C. Barnes, M. Solinas, SR Goldberg: Prior exposure to THC increases the addictive effects of nicotine in rats. In: Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. Volume 38, number 7, June 2013, pp. 1198–1208, doi: 10.1038 / npp.2013.16 , PMID 23314220 , PMC 3656362 (free full text).
- ↑ Stephen J. Kohut: Interactions between nicotine and drugs of abuse: a review of preclinical findings. In: The American Journal of Drug and Alcohol Abuse. 43, 2016, p. 155, doi : 10.1080 / 00952990.2016.1209513 (review) ( free full text ).
- ↑ K. MacDonald, K. Pappas: WHY NOT POT ?: A Review of the Brain-based Risks of Cannabis. In: Innovations in clinical neuroscience. Volume 13, Number 3-4, 2016 Mar-Apr, pp. 13-22, PMID 27354924 , PMC 4911936 (free full text) (review).
- ↑ LV Panlilio, Z. Just Inova: Preclinical Studies of cannabinoid Reward, treatments for cannabis use disorder, and Addiction-Related Effects of cannabinoid exposure. In: Neuropsychopharmacology . Volume 43, number 1, January 2018, pp. 116-141, doi : 10.1038 / npp.2017.193 , PMID 28845848 , PMC 5719102 (free full text) (review).
- ↑ DP Covey, JM Wenzel, JF Cheer: Cannabinoid modulation of drug reward and the implications of marijuana legalization. In: Brain Research . Volume 1628, Pt A December 2015, pp. 233–243, doi : 10.1016 / j.brainres.2014.11.034 , PMID 25463025 , PMC 4442758 (free full text) (review).
- ↑ J. Renard, WJ Rushlow, SR Laviolette: What Can Tell Us about council Adolescent Cannabis Exposure? Insights from Preclinical Research. In: Canadian journal of psychiatry. Revue canadienne de psychiatrie. Volume 61, number 6, 06 2016, pp. 328–334, doi : 10.1177 / 0706743716645288 , PMID 27254841 , PMC 4872245 (free full text) (review).
- ↑ D. Kandel: Stages in adolescent involvement in drug use. In: Science. Volume 190, Number 4217, November 1975, pp. 912-914, PMID 1188374 .
- ↑ K. Yamaguchi, DB Kandel: Patterns of drug use from adolescence to young adulthood: II. Sequences of progression. In: American Journal of Public Health . Volume 74, Number 7, July 1984, pp. 668-672, PMID 6742252 , PMC 1651663 (free full text).
- ↑ D. Kandel, K. Yamaguchi: From beer to crack: developmental patterns of drug involvement. In: American Journal of Public Health . Volume 83, Number 6, June 1993, pp. 851-855, PMID 8498623 , PMC 1694748 (free full text).
- ↑ DB Kandel (Ed.): Stages and Pathways of Drug Involvement: Examining the Gateway Hypothesis , Cambridge University Press, 2002, ISBN 978-0-521-78969-1 , p. 4.
- ^ National Academies of Sciences, Engineering, and Medicine. Health and Medicine Division. Board on Population Health and Public Health Practice. Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems: Public Health Consequences of E-Cigarettes . In: National Academies of Sciences . 2018. doi : 10.17226 / 24952 . PMID 29894118 . (Review) (free full text), (in the original: "There is substantial evidence [emphasis in the original] that e-cigarette use increases risk of ever using combustible tobacco cigarettes among youth and young adults.")
- ^ DM Lydon, SJ Wilson, A. Child, CF Geier: Adolescent brain maturation and smoking: what we know and where we're headed. In: Neuroscience and biobehavioral reviews. Volume 45, September 2014, pp. 323-342, doi : 10.1016 / j.neubiorev.2014.07.003 , PMID 25025658 , PMC 4451244 (free full text) (review).
- ^ M. Yuan, SJ Cross, SE Loughlin, FM Leslie: Nicotine and the adolescent brain. In: The Journal of Physiology. Volume 593, number 16, August 2015, pp. 3397-3412, doi : 10.1113 / JP270492 , PMID 26018031 , PMC 4560573 (free full text) (review).
- ^ WM Cox, E. Klinger, JS Fadardi: Free will in addictive behaviors: A matter of definition. In: Addictive behaviors reports. Volume 5, June 2017, pp. 94-103, doi: 10.1016 / j.abrep.2017.03.001 , PMID 29450231 , PMC 5800588 (free full text) (review), PDF .
- ↑ Best addiction prevention: Strengthening the child's personality. (No longer available online.) In: psychologie.at. Archived from the original on February 24, 2009 ; accessed on February 2, 2014 .
- ↑ a b Peter Degkwitz: Dependency or self-determined individual? Comment on the debate about understanding drug use and addiction. In: Accepting Drug Work. 1999, p. 38.
- ↑ M. Farrell, J. Ward, R. Mattick, W. Hall, G. Stimson, D. des Jarlais and others. a .: Methadone maintenance treatment in opiate dependence: a review. In: BMJ. 309, 1994, pp. 997-1001.
- ↑ Freye: Opioids in Medicine. 8th edition. Springer, 2010.
- ↑ M. Daglish, A. Lingford-Hughes, D. Nutt: Human functional neuroimaging connectivity research in dependence. In: Reviews in the neurosciences. Volume 16, Number 2, 2005, pp. 151-157, PMID 15957578 (review).
- ↑ T. Kienast, J. Wrase, A. Heinz: [Neurobiology of substance-related addiction: findings of neuroimaging]. In: Advances in neurological psychiatry. Volume 76 Suppl 1, May 2008, pp. S68-S76, doi: 10.1055 / s-2008-1038141 , PMID 18461548 (review).
- ↑ KD Ersche, GB Williams, TW Robbins, ET Bullmore: Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. In: Current opinion in neurobiology. Volume 23, number 4, August 2013, pp. 615-624, doi: 10.1016 / j.conb.2013.02.017 , PMID 23523373 (free full text) (review).
- ↑ CE Yesalis, JR Vicary, WE Buckley, AL dispute, DL Katz, JE Wright: Indications of psychological dependence among anabolic-androgenic steroid abusers. In: NIDA research monograph. Vol. 102, 1990, pp. 196-214, PMID 2079973 (review).
