Ixekizumab
| Ixekizumab | ||
|---|---|---|
| Mass / length primary structure | 146.2 kDa | |
| Identifier | ||
| External IDs |
|
|
| Drug information | ||
| ATC code | L04 AC13 | |
| DrugBank | DB11569 | |
| Drug class | Monoclonal antibody , immunosuppressant | |
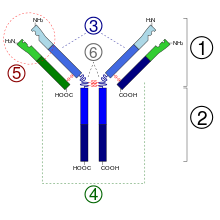
1. Fab section
2. Fc section
3. heavy chains
4. light chains
5. antigen binding site ( paratope )
6. hinge region
(*) -SS disulfide bridge
Ixekizumab is a drug for the treatment of psoriasis (psoriasis) and psoriatic arthritis . The humanized monoclonal antibody is produced recombinantly in CHO cells .
properties
Ixekizumab is a humanized immunoglobulin G of subclass 4 (IgG 4 ). It consists of two identical long ("heavy") protein chains of 445 amino acids each and two identical short ("light") protein chains of 219 amino acids each, which together make up the protein's molar mass of 146,158 Daltons . The heavy chains are depending on Asn -296 with an oligosaccharide glycosylated . The chains are connected to one another via disulfide bridges .
By exchanging serine in the joint region at position 227 (S-227) by proline , the dissociation into two half-antibodies is suppressed. The lack of terminal lysine (K-447) found in IgG 4 wild type also contributes to the structural stability of the homodimer.
Mechanism of action
Ixekizumab binds selectively and with high affinity to interleukin-17 A (IL-17A), a pro-inflammatory cytokine that has been associated with inflammatory and autoimmune diseases such as psoriasis. The biologically active form of IL-17A includes both the homodimer IL-17A and the heterodimer IL-17AF. Increased IL-17A concentrations stimulate the proliferation and activation of keratinocytes in the skin . IL-17A also induces the production of other cytokines and prostaglandins and plays a role in the activation of CD4- positive cells. The neutralization of IL-17A by ixekizumab inhibits these processes.
Ixekizumab does not neutralize the other subtypes of the interleukin-17 family IL-17B to F.
application areas
Ixekizumab has been approved in the US since March 2016 and in the EU since April 2016 under the name Taltz for the systemic treatment of adult patients with moderate to severe plaque psoriasis. The drug is administered subcutaneously and can be injected by the patient himself with a pen . After a loading dose, the drug is given every two weeks up to week 12 and every four weeks thereafter. Taltz was developed by Eli Lilly .
Ixekizumab was approved by the US Food and Drug Administration (FDA) for the United States in December 2017 for the indication of psoriatic arthritis . EU approval for this indication took place in January 2018.
Admission Studies
Ixekizumab was tested for safety and efficacy in three randomized, double-blind clinical trials with a total of 3,866 participants with plaque psoriasis and was found to be superior to placebo or etanercept .
A PASI -75 response and a sPGA of 0 (“ no symptoms ”) or 1 (“almost no symptoms ”) after 12 weeks were used as the combined primary endpoint in all studies .
Side effects and restrictions on use
The most common side effects were reactions at the injection site and infections of the upper respiratory tract - especially Naso pharyngitis observed - (inflammation of the nasal and pharyngeal mucosa). Treatment with ixekizumab also results in an increased infection rate for oral candidiasis , conjunctivitis, and tinea infections.
Ixekizumab must not be used in the presence of active tuberculosis ; Caution should be exercised with other clinically relevant infections and inflammatory bowel disease. Simultaneous use with live vaccines or use during pregnancy is not recommended .
Pharmacokinetics
After a loading dose and an initial dosing schedule of once every two weeks, steady state is reached after eight weeks .
The average bioavailability is between 54% and 90%. The mean plasma half-life is 13 days.
Preparation name (s)
Lilly : Taltz (USA, EU)
Web links
Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Ixekizumab
Individual evidence
- ↑ Public assessment report on Taltz ( EPAR ) of the European Medicines Agency from February 25, 2016.
- ↑ a b c d e Taltz product information , as of May 2, 2016, website of the European Medicines Agency.
- ↑ Lilly's Taltz® (ixekizumab) Receives US FDA Approval for the Treatment of Active Psoriatic Arthritis ( Memento of the original from December 5, 2017 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , PM Lilly, December 1, 2017, accessed December 5, 2017.
- ↑ https://www.pharmazeutische-zeitung.de/2018-01/zulassungserweiterung-ixekizumab-auch-bei-psoriasis-arthritis/