Ramucirumab
| Ramucirumab | ||
|---|---|---|
|
||
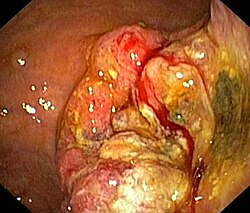
| Already advanced stage of stomach cancer | ||
| Mass / length primary structure | 1320 amino acids | |
| Drug information | ||
| DrugBank | DB05578 | |
| Drug class | Monoclonal antibody | |
Ramucirumab ( IMC-1121B ; trade name Cyramza ) is a monoclonal antibody that is used in the treatment of advanced or metastatic gastric cancer , colorectal cancer , non-small cell lung cancer, or hepatocellular carcinoma .
history
Cyramza was approved by the American health authority FDA in April 2014, with manufacture by the pharmaceutical company Eli Lilly . The assessment of this newly developed drug for cancer therapy was based on an accelerated approval process .
In Germany (and the EU), Cyramza is approved for the treatment of certain patients with stomach, colon, lung and liver cancer.
Properties of the molecule and its manufacturing process
Ramucirumab is a monoclonal antibody IgG1 consisting of two heavy chains (engl. Heavy chains ; type γ) and two light chains (engl. Light chains , κ type). This antibody consists of a total of 1320 amino acids and has a molar mass of approx. 146.8 kDa . This antibody is produced by the mouse myeloma cell line NS0.
Effective range
Tumors need blood vessels to supply nutrients and oxygen so that they can divide and spread further. Vascular Endothelial Growth Factor (VEGF) is an important molecule in the formation and growth of new blood vessels ( angiogenesis ), whereby this protein is upregulated especially in cancer cells. The tyrosine kinase Vascular endothelial growth factor receptor 2 (VEGF R2) is a key mediator of VEGF-induced blood vessel growth. The binding of ramucirumab to the extracellular domain of the VEGF R2 receptor prevents the ligands VEGF-A, VEGF-C and VEGF-D from binding to this VEGF R2 receptor, which also prevents the growth of new blood vessels and thus prevents the tumor from being supplied Nutrients is prevented.
administration
Ramucirumab is given as an infusion at a dose of 8 mg / kg body weight over a period of 60 minutes every two weeks.
See also
- Nomenclature of monoclonal antibodies , convention for naming monoclonal antibodies
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Ramucirumab
Individual evidence
- ↑ Cyramza. EMA, September 17, 2018, accessed December 5, 2019 .
- ↑ a b FDA approves Cyramza for stomach cancer. FDA, accessed August 25, 2014 .
- ↑ Sven Siebenand: Liver cancer: second-line therapy with ramucirumab possible. Pharmaceutical newspaper , September 16, 2019, accessed December 5, 2019 .
- ↑ a b CLINICAL PHARMACOLOGY AND BIOPHARMACEUTICS REVIEW (S). FDA, accessed August 25, 2014 .
- ↑ Saharinen Pipsa: VEGF and angiopoietin signaling in tumor angiogenesis and metastasis . In: Trends in Molecular Medicine . 17, No. 7, July 2011, pp. 347-362. doi : 10.1016 / j.molmed.2011.01.015 .
- ↑ Carmeliet P .: VEGF as a key mediator of angiogenesis in cancer . In: Oncology (Basel) . 69, November 2005, pp. 4-10. doi : 10.1159 / 000088478 .
- ↑ HIGHLIGHTS OF PRESCRIBING INFORMATION. (pdf) FDA, accessed on August 25, 2014 (English).