Homozygous familial hypercholesterolemia
| Classification according to ICD-10 | |
|---|---|
| E78 | Disorders of lipoprotein metabolism and other lipidemias |
| E78.0 | Pure hypercholesterolemia |
| ICD-10 online (WHO version 2019) | |
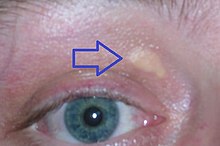
The homozygous familial hypercholesterolemia (HoFH) is a hereditary , rare disease . As a form of familial hypercholesterolemia, it is one of the lipid metabolism disorders . Patients with HoFH show a massive increase in low density lipoprotein (LDL), a cholesterol fraction in the blood. Due to the high LDL cholesterol level, the lipid can be deposited in the skin and tendons over the long term, which can result in xanthomas (yellowish, nodular deposits of lipids in the skin). The lipid is also deposited in the vessel walls and causes severe arteriosclerosis that sets in early with a significantly reduced life expectancy.
HoFH must be differentiated from the much more common, heterozygous familial hypercholesterolemia (HeFH), which is also associated with a significantly increased risk of premature cardiovascular events, but is less dramatic.
frequency
In the literature, a disease frequency ( prevalence ) of HoFH of about 1: 1,000,000 is assumed, i.e. H. there is one affected person for every 1,000,000 members of a population. However, a higher prevalence can be expected in some populations. A disease is considered rare if it occurs with a prevalence of 1: 2000, i.e. at most in every two thousandth member of the population.
For comparison: the heterozygous form of familial hypercholesterolemia (HeFH) has an estimated prevalence of 1: 500. It is considered to be the most common monogenic disease. More recent population studies even assume an incidence of 1: 200.
root cause
Familial hypercholesterolemia is inherited as an autosomal dominant trait . However, those affected can have mutations in different genes with the same phenotype . In homozygous individuals, defective alleles (manifestations of a gene) are inherited from both parents. Therefore, the disease shows a particularly strong expression: As a rule, the binding of LDL cholesterol to the receptors is severely impaired.
The most common (85-90%) are inherited genetic defects that impair the function of the LDL receptor , which is responsible for removing LDL cholesterol from the blood. The mutation can be at over 1,600 gene loci (gene positions).
Other causes can include mutations in the gene for apolipoprotein B, an important structural element of LDL cholesterol, or in the gene for PCSK9 (proprotein convertase subtilisin / kexin type 9), an enzyme that plays an important role in the breakdown of LDL receptors, be. In rare cases, there is an autosomal recessive mutation in the LDL receptor adapter protein 1 (LDLRAP1) that corresponds to the HoFH phenotype in a homozygous form.
People with HoFH can have two identical mutations (classically homozygous), two different mutations in the same gene ( compound heterozygous ), two different mutations in two different genes (doubly heterozygous) or two mutations in the autosomal recessive LDL receptor AP1 gene ( autosomal recessive hypercholesterolemia (ARH)). In contrast, heterozygous familial hypercholesterolemia is caused by the inheritance of a mutated LDL receptor allele.
Symptoms and diagnosis
The clinical diagnosis of HoFH is typically made based on a combination of the following criteria:
- Massively increased total cholesterol values from 17 to 26 mmol / l (650 to 1000 mg / dl) and LDL cholesterol values> 13 mmol / l (> 500 mg / dl)
- Incidence of xanthomas (cutaneous or tendinous) in childhood.
- Signs of arteriosclerosis, i.e. diseases and symptoms caused by vascular problems, e.g. B. peripheral arterial occlusive disease , narrowing of the coronary arteries up to myocardial infarction , stroke .
- Corresponding problems in the blood relatives (positive family history )
Genetic confirmation of the HoFH diagnosis can also be helpful. Functional mutation (s) on both LDL receptor alleles or alleles that influence the LDL receptor functionality (Apo B, PCSK9 or ARH) can be documented. Nevertheless, in 20 to 60 percent of cases, despite a clinical diagnosis, no change in the genetic material (mutation) can be found with the current genetic diagnosis. It can be assumed that not all HoFH patients can be unequivocally diagnosed by examining the known genes.
therapy
Therapy goal and principles
The main goal of HoFH therapy is a permanent lowering of the LDL cholesterol in the blood. The therapy is based on the joint guidelines of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) for the treatment of disorders of lipid metabolism. For patients with familial hypercholesterolemia, regardless of whether they are homozygous or heterozygous hypercholesterolemia, they define an LDL cholesterol target value <100 mg / dl (target value for individuals at high cardiovascular risk) and values for patients with familial hypercholesterolemia and the presence of cardiovascular disease <70 mg / dl (target value for individuals at very high risk).
If possible, patients should be treated in a specialized center (lipid outpatient clinic or apheresis center) with lipid-lowering drugs and LDL apheresis. The treatment concept also includes a low-fat, low-cholesterol diet, nicotine abstinence and the recommendation for regular physical activity.
Drug therapy options
HoFH patients do not respond adequately to previously available lipid-lowering pharmacotherapies. Two studies described an average reduction in LDL cholesterol of seven percent with statins . Therapy with high-dose statins (simvastatin, predominantly atorvastatin 80 mg, rosuvastatin 40 mg), in 50% of the patients in combination with ezetimibe , achieved an approx. 26 percent reduction regardless of the form of therapy.
Since 2013, the active ingredient lomitapid has been approved in Europe in combination with a low-fat diet and other lipid-lowering drugs with or without LDL apheresis in adult patients with homozygous familial hypercholesterolaemia. (For familial chylomicronemia , lomitapid was granted orphan drug status by the European Medicines Agency (EMA) in December 2010, but no approval was granted for this indication. ) It comes from the selective inhibition of microsomal triglyceride transfer protein (MTP) there is a reduced formation of lipid complexes ( Very Low Density Lipoprotein (VLDL) in the liver, chylomicrons in the intestine). As a result, VLDL is no longer released from the liver into the blood or chylomicrons are no longer absorbed from the intestine. This results in a lowering of the blood levels of VLDL, LDL, chylomicrons and apolipoprotein B (Apo B).
Mipomersen is an antisense - oligonucleotide which coding B-Apo to the messenger RNA binds, thereby leading to a reduced formation of Apo B in the liver and correspondingly to a decrease in LDL cholesterol. The drug was approved in the USA by the Food and Drug Administration (FDA) under the trade name Kynamro ® for the treatment of patients with homozygous FH under special conditions. The approval was rejected by the European Commission in 2012 and again in 2013 because the risks and side effects evident from the studies (a high proportion of patients discontinued use because of the side effects, increase in transaminases, obesity of the liver, increased cardiovascular disease compared to the placebo group Problems) outweigh the possible advantages of the active ingredient, especially with regard to the necessary long-term use.
For patients with FH and high cardiovascular risk, two fully human antibodies against the PCSK9 protein have been available since the end of 2015, the PCSK9 inhibitors alirocumab (trade name: Praluent) and evolocumab (Repatha) to lower LDL cholesterol. Another PCSK9 inhibitor ( evinacumab ) is currently still in clinical development . The manufacturing company Regeneron Pharmaceuticals ( Sanofi ) has received breakthrough therapy status from the US Food and Drug Administration (FDA) for evinacumab for the treatment of patients with homozygous familial hypercholesterolemia.
Volanesorsen is an antisense inhibitor of apolipoprotein (apo) C-III and is being investigated in both patients with hypertriglyceridemia and in familial chylomicronemia (FCS). FCS is also a rare, autosomal recessive inherited disorder, which is counted as an "orphan disease" and is caused by decreased or absent lipoprotein lipase activity (LPL). FCS is characterized by a significant accumulation of chylomicrons and extreme hypertriglyceridemia, usually> 1,000 mg / dL or 11 mmol / L.
LDL apheresis
The LDL apheresis is the dialysis similar process which is carried out every one to two weeks, and the LDL-cholesterol wash out extracorporeally from the blood. This is offered in around 170 centers in Germany and the costs for this therapy are covered by the health insurance companies upon request. In the long run, the treatment can lead to a 40 to 50 percent reduction in LDL cholesterol. Despite the mentioned reduction through apheresis, the LDL cholesterol values in HoFH patients are still well above the published target values of <100 mg / dl on average over time. It is assumed that even with LDL apheresis, cardiovascular disease cannot in most cases be prevented, but only postponed.
Course and prognosis
In HoFH patients, there is a direct relationship between the level of LDL cholesterol and the risk of cardiovascular disease events. HoFH patients therefore often have to undergo interventions on the vascular system ( catheter expansion , possibly with the use of a stent , bypass operation ) at an early stage, as teenagers or young adults .
In a retrospective study published in 2011, HoFH patients only reached an average age of 33 years with maximum lipid-lowering drug therapy. A 2012 HoFH study on additional apheresis therapy found that 86 percent of patients progressed cardiovascular disease despite this treatment.
literature
- FJ Raal, RD Santos: Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. In: Atherosclerosis. Volume 223, Number 2, August 2012, pp. 262-268, ISSN 1879-1484 . doi: 10.1016 / j.atherosclerosis.2012.02.019 . PMID 22398274 . (Review).
- S. Walzer, K. Travers et al .: Homozygous familial hypercholesterolemia (HoFH) in Germany: an epidemiological survey. In: ClinicoEconomics and outcomes research: CEOR. Volume 5, 2013, pp. 189-192, ISSN 1178-6981 . doi: 10.2147 / CEOR.S43087 . PMID 23662069 . PMC 3647446 (free full text).
Individual evidence
- ↑ National Institute of Health (NIH): List of rare diseases (Familial hyperlipo-proteinemia type 1)
- ↑ a b c d e f g h i j AL. Catapano et al .: ESC / EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). In: European heart journal. Volume 37, 2016, pp. 2999–3058, doi: 10.1093 / eurheartj / ehw272 .
- ↑ a b c d P. N. Hopkins, PP Toth et al .: Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. In: Journal of Clinical Lipidology . Volume 5, Number 3 Suppl, June 2011, pp. S9-S17, ISSN 1933-2874 . doi: 10.1016 / j.jacl.2011.03.452 . PMID 21600530 .
- ↑ a b c d e f F. J. Raal, RD Santos: Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. In: Atherosclerosis. Volume 223, Number 2, August 2012, pp. 262-268, ISSN 1879-1484 . doi: 10.1016 / j.atherosclerosis.2012.02.019 . PMID 22398274 . (Review).
- ↑ BG Nordestgaard, MJ Chapman, among others: Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for Clinicians to prevent coronary heart disease: Consensus Statement of the European Atherosclerosis Society. In: European heart journal. Volume 34, Number 45, December 2013, pp. 3478-3490, ISSN 1522-9645 . doi: 10.1093 / eurheartj / eht273 . PMID 23956253 . PMC 3844152 (free full text).
- ^ W. Siegenthaler, HE Blum (Ed.): Clinical Pathophysiology. 9th edition. Georg Thieme Verlag, 2006.
- ↑ a b c d A. C. Goldberg, PN Hopkins et al .: Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. In: Journal of clinical lipidology. Volume 5, Number 3 Suppl, June 2011, pp. S1-S8, ISSN 1933-2874 . doi: 10.1016 / j.jacl.2011.04.003 . PMID 21600525 .
- ^ AD Marais: Familial hypercholesterolaemia. In: Clin Biochem Rev. 25 (1), 2004, pp. 49-86.
- ↑ PJ Talmud, S. Shah et al .: Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: a case-control study. In: Lancet. Volume 381, Number 9874, April 2013, pp. 1293-1301, ISSN 1474-547X . doi: 10.1016 / S0140-6736 (12) 62127-8 . PMID 23433573 .
- ^ A b G. R. Thompson, M. Barbir et al .: Efficacy criteria and cholesterol targets for LDL apheresis. In: Atherosclerosis. Volume 208, Number 2, February 2010, pp. 317-321, ISSN 1879-1484 . doi: 10.1016 / j.atherosclerosis.2009.06.010 . PMID 19589528 . (Review).
- ↑ C. Gagné, D. Gaudet, E. Bruckert: Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. In: Circulation. Volume 105, Number 21, May 2002, pp. 2469-2475, ISSN 1524-4539 . PMID 12034651 .
- ↑ a b F. J. Raal, GJ Pilcher et al: Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. In: Circulation. Volume 124, Number 20, November 2011, pp. 2202-2207, ISSN 1524-4539 . doi: 10.1161 / CIRCULATIONAHA.111.042523 . PMID 21986285 .
- ↑ European Medicines Agency (EMA): Lojuxta / Lomitapide: EPAR summary for the public
- ↑ European Medicines Agency (EMA): orphan designation (EU / 3/10/823)
- ↑ MM Hussain, P. Rava et al: Multiple functions of microsomal triglyceride transfer protein. In: Nutrition & metabolism. Volume 9, 2012, p. 14, ISSN 1743-7075 . doi: 10.1186 / 1743-7075-9-14 . PMID 22353470 . PMC 3337244 (free full text).
- ↑ ema.europa.eu Assessment report Kynamro, EMA Committee for Medicinal Products for Human Use (CHMP), July 2013, accessed on February 3, 2014.
- ↑ egeneron Announces Evinacumab has Received FDA Breakthrough Therapy Designation for Homozygous Familial Hypercholesterolemia (HoFH) , PM Regeren, April 6, 2077, accessed September 8, 2017
- ↑ a b L. C. Hudgins, BR Gordon, TS Parker, SD Saal, DM Levine, AL Rubin: LDL Apheresis: an effective and safe treatment for refractory hypercholesterolemia. In: Cardiovasc Drug Rev . 20, 2002, pp. 271-280.
- ↑ a b A. Græsdal, MP Bogsrud et al .: Apheresis in homozygous familial hypercholesterolemia: the results of a follow-up of all Norwegian patients with homozygous familial hypercholesterolemia. In: Journal of clinical lipidology. Volume 6, Number 4, 2012 Jul-Aug, pp. 331-339, ISSN 1933-2874 . doi: 10.1016 / j.jacl.2012.03.004 . PMID 22836070 .
- ↑ JL Goldstein, HH Hobbs et al: Familial Hypercholesterolemia. McGraw-Hill, New York 2001.
- ↑ S. Moorjani, M. Roy, among others: Mutations of low-density lipoprotein receptor gene, variation in plasma cholesterol, and expression of coronary heart disease in homozygous familial hypercholesterolaemia. In: Lancet. Volume 341, Number 8856, May 1993, pp. 1303-1306, ISSN 0140-6736 . PMID 8098448 .


