Ezetimibe
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Ezetimibe | ||||||||||||||||||
| other names |
(3 R , 4 S ) -1- (4-fluorophenyl) -3 - [(3 S ) -3- (4-fluorophenyl) -3-hydroxypropyl] -4- (4-hydroxyphenyl) azetidin-2-one ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 24 H 21 F 2 NO 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class |
Cholesterol absorption inhibitors |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 409.43 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
164-166 ° C |
||||||||||||||||||
| solubility |
1 mg l −1 in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ezetimibe (manufacturer: MSD Sharp & Dohme ) is a drug from the group of azetidones and inhibits the absorption of cholesterol on the brush border of the villi cells of the small intestine .
The cholesterol absorption inhibitor ezetimibe has been marketed under the trade name Ezetrol by MSD / Essex in Germany since 2002.
synthesis
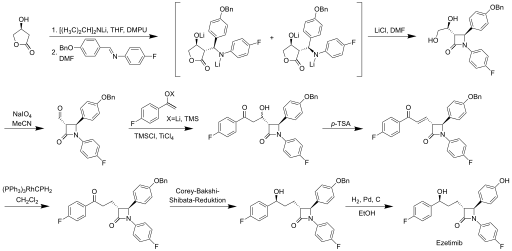
Various synthetic routes have been developed for the production of ezetimibe. A synthesis developed at the Schering-Plow Research Institute starts from commercially available 3- ( S ) -hydroxy-γ-butyrolactone as the chiral pool building block.
In the central first step, the addition of lithium diisopropylamide at −35 ° C in a mixture of dimethylpropyleneurea and dimethylformamide generates the dianion of the lactone . Further addition of an aryl-substituted imine forms the trans - lactam in good yield and high enantio- and diastereoselectivity . In the next step, the diol side chain is converted into the aldehyde by oxidation with sodium periodate . The aldehyde is converted into a hydroxyketone with 4-fluoroacetophenone with the addition of lithium diisopropylamide in a Mukaiyama aldol reaction . An enone is formed by dehydration with p -toluenesulfonic acid . This is converted into the chiral alcohol via a Corey-Bakshi-Shibata reduction . After removing the protective group of the phenol, the target product ezetimibe is produced in the last step.
application areas
According to the product information, ezetimibe is approved for the following areas of application:
Primary hypercholesterolemia
Ezetimibe is indicated in combination with an HMGCoA reductase inhibitor (statin) as a dietary supplement for use in patients with primary (heterozygous familial and non-familial) hypercholesterolaemia for whom therapy with a statin alone is insufficient.
Monotherapy
Ezetimibe monotherapy is indicated as an adjunct to diet for use in patients with primary (heterozygous familial and non-familial) hypercholesterolaemia for whom a statin is considered inappropriate or not tolerated.
Homozygous familial hypercholesterolemia (HoFH)
Ezetimibe is indicated for use with a dietary statin in patients with homozygous familial hypercholesterolaemia . Patients can receive other concomitant therapies (such as LDL apheresis).
Homozygous Sitosterinemia (Phytosterinemia)
Ezetimibe is indicated as an adjunct to diet for use in patients with homozygous familial sitosterinaemia .
Clinical information
Use during pregnancy and breastfeeding
No clinical data are available on use during pregnancy and breastfeeding or in children under 10 years of age.
Special patient groups (diabetics, kidney patients)
A 12-fold increase in the plasma level of ezetimibe was observed following concomitant administration of ciclosporin in a kidney transplant patient with severe renal impairment .
Adverse effects (side effects)
The most common side effects of ezetimibe with monotherapy are gastrointestinal discomfort, tiredness and headache. In addition, allergic reactions and transaminase increases are possible.
The side effects of the simvastatin / ezetimibe combination were on a par with simvastatin monotherapy, but the SEAS study saw an increase in cancer cases with ezetimibe.
Pharmacological properties
Mechanism of action (pharmacodynamics)
Ezetimibe completely inactivates the cholesterol transporter NPC1L1 . The active substance is converted in the small intestine by UDP-glucuronosyltransferases into its β- glucuronide , which inhibits the absorption of cholesterol even more than ezetimibe itself. The glucuronide is absorbed in the small intestine and returns to the small intestine with the bile ( enterohepatic circulation ). As a result, cholesterol (exogenous) and cholesterol excreted in the bile (biliary endogenous) is absorbed to 50% less via the small intestine. By increasing the endogenous cholesterol production in the liver, the effect is ultimately reduced to a max. 20% lowering of serum LDL cholesterol levels.
With a simultaneous reduction in the triglyceride level of 5.7% and an only slightly pronounced increase in the HDL concentration, the effect is therefore less than after the administration of statins , so that ezetimibe has so far only been used as a reserve drug in the event of an intolerance to statins or absorption inhibitors such as colestyramine you can see. However, the combination with statins leads to a further significant reduction in LDL values as a surrogate parameter for serious cardiovascular diseases .
Studies
Immediately after the market launch of ezetimibe (trade name Ezetrol ) in November 2002, ezetimibe was evaluated by the drug telegram as follows:
- Ezetimibe lowers serum cholesterol by blocking the absorption of cholesterol from the intestine with a mechanism of action that differs from previous lipid-lowering drugs.
- Long-term studies on clinical benefit and safety are lacking.
- In the rare familial forms of hypercholesterolaemia and sitosterolaemia, ezetimibe could become a reserve status. The therapeutic value for this patient group remains to be clarified in further studies with clinically relevant endpoints.
Up to 2010 there were no studies that could show a reduction in disease incidence and mortality . No published study lasted longer than three months; only indirect symptoms (surrogate parameters) such as blood values or the thickness of the artery wall (intima-media thickness) were examined . One example is the EASE study ( E zetimibe A dd-On to S tatin for E ffectiveness) in 3030 previously inadequately treated patients: 2010 of them received statin plus ezitimibe or 1020 statin plus placebo for six weeks. There was a significant reduction in LDL cholesterol of 26% versus 3%. The aim of treatment was an LDL cholesterol level of 95 mg / dl. This achieved 21% with placebo and 71% with ezetimibe.
In the final report that the Institute for Quality and Efficiency in Health Care presented to the G-BA on September 12, 2011 (8 years and 10 months after the introduction of ezetimibe on the German market), the conclusion is as in the preliminary report: “It There is no proof of benefit or harm from treating patients with hypercholesterolemia with ezetimibe compared to treatment with placebo. This applies to both monotherapy and combination therapy. No studies were available for monotherapy. ”The report also contained the following:“ High blood cholesterol levels are considered a risk factor for heart attacks and other cardiovascular diseases. However, this does not necessarily mean that every drug that lowers cholesterol can also prevent heart attacks [...] In particular, there is a lack of evidence that patients benefit more if they take ezetimibe in addition to statins for heart attack prevention. "
The publication of the ENHANCE study was delayed for a long time. In response to public and political pressure, some results of the ENHANCE study were pre-published by the ezetimibe manufacturers in the USA on January 14, 2008 : There was no significant difference between simvastatin / ezetimibe and simvastatin alone in terms of the primary endpoint - the intima-media thickness . Only a greater reduction in LDL levels compared to simvastatin monotherapy was achieved; However, this was not a primary study goal.
A long-term study to assess security and long-term benefit, the IMPROVE-IT study (started in October 2005), was completed in 2015. It was a double-blind, placebo-controlled, randomized study on 18,144 over 50-year-old high-risk patients from 39 countries. The study participants did not have any hypercholesterolemia at the start of the study, their LDL cholesterol had to be between 50 and 125 mg / dl. The addition of the active ingredient ezetimibe to statin therapy after an acute coronary syndrome reduced the patient's LDL value by up to 23%. In terms of mortality among study participants, ezetimibe had no advantage; the incidence rates for cardiovascular death, for non-fatal myocardial infarction, for re-hospitalization for unstable angina, for revascularization and for non-fatal stroke all fell significantly by 6.4% compared to the control group .
Austria
In Austria, the costs of the prescription are covered by the health insurance companies, provided that the LDL remains higher than 113 mg / dl in patients with clinically manifest arteriosclerosis ( CHD ) and / or diabetes mellitus while statin therapy is ongoing .
Trade names
Ezetrol (D, A, CH)
- with simvastatin : Inegy (D, A, CH), Goltor
- with atorvastatin : Atozet, Tioblis
Web links
- Alfred Klement: New therapy principle for CHD: Cholesterol absorption inhibition with Ezetrol® . In: Österreichische Apotheker-Zeitung . 59th year, no. 2 , January 12, 2005, p. 69–70 ( apoverlag.at [PDF; 1.9 MB ; accessed on September 8, 2010]).
- Key protein for ezetimibe's mechanism of action discovered. In: Journal Med, information for doctors. rs media GmbH, March 22, 2004, accessed on September 8, 2010 (source: MSD).
Individual evidence
- ^ The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. 2006, ISBN 0-911910-00-X , p. 668.
- ↑ K. Takács-Novák, M. Urac, P. Horváth, G. Völgyi, BD Anderson, A. Avdeef: Equilibrium solubility measurement of compounds with low dissolution rate by Higuchi's Facilitated Dissolution Method. A validation study. In: Eur. J. Pharm. Sci. 106, 2017, pp. 133-144, doi: 10.1016 / j.ejps.2017.05.064 .
- ↑ There is not yet a harmonized classification for this substance . A label of (3R, 4S) -1- (4-fluorophenyl) -3 - ((S) -3- (4-fluorophenyl) -3-hydroxypropyl) -4- (4- (4-), derived from a self-classification by the distributor hydroxyphenyl) azetidin-2-one in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 10, 2020.
- ↑ Guangzhong Wu, YeeShing Wong, Xing Chen, Zhixian Ding: A Novel One-Step Diastereo- and Enantioselective Formation of -Azetidinones and Its Application to the Total Synthesis of Cholesterol Absorption Inhibitors. In: The Journal of Organic Chemistry. 64, 1999, pp. 3714-3718. doi: 10.1021 / jo990428k .
- ↑ a b Ezetimibe (Ezetrol®) - Information from the KBV in the context of Section 73 (8). (221 kB; PDF) In: Agent AKTUELL 03/2003 (updated). KBV in cooperation with AkdÄ, November 1, 2005, p. 2 , accessed on September 8, 2010 : "No clinical data are available on use during pregnancy and breastfeeding or in children under 10 years of age" " .
- ↑ Yellow List Online: Ezetimibe - Use, Effect, Side Effects | Yellow list. Retrieved June 20, 2019 .
- ↑ Richard Daikeler, idols Use, Sylke Waibel: diabetes. Evidence-based diagnosis and therapy. 10th edition. Kitteltaschenbuch, Sinsheim 2015, ISBN 978-3-00-050903-2 , p. 150.
- ↑ John JP Kastelein et al .: Simvastatin with or without Ezetimibe in Familial Hypercholesterolemia . In: N Engl J Med . No. 358 , 2008, p. 1431-1443 ( Article ).
- ^ Richard Peto et al .: Analyzes of Cancer Data from Three Ezetimibe Trials . In: N Engl J Med . 2008 ( article ).
- ↑ Entry on ezetinib. In: Römpp Online . Georg Thieme Verlag, accessed on July 1, 2019.
- ↑ drug telegram . No. 11, 2002, New To The Market, The Cholesterol Lowering Agent Ezetemibe (Ezetrol).
- ↑ Amendment to the Drugs Directive (AM-RL) in Appendix IV: Therapy information on ezetimibe dated December 17, 2009 . In: Joint Federal Committee (Ed.): Deutsches Ärzteblatt . tape 107 , no. 15 , April 16, 2010, p. A-725 ( aerzteblatt.de [PDF; 87 kB ; accessed on September 8, 2010]): "Studies that examined the effects on mortality or morbidity are not published on ezetimibe"
- ↑ Large study confirms the benefit of ezetimibe. In: Doctors newspaper. March 12, 2004, accessed on September 8, 2010 : "No patient was satisfactorily adjusted to this therapy, measured against the target values for lowering cholesterol recommended in the US guidelines, at the start of the study" .
- ↑ Ezetimibe in hypercholesterolemia, IQWiG, short version of the final report, version 1.0 (July 18, 2011).
- ↑ Lipid-lowering drug ezetimibe fails in endpoint study. Deutsches Ärzteblatt online, January 15, 2008.
- ↑ Merck / Schering-Plow Pharmaceuticals Provides Results of the ENHANCE Trial. Press release, January 14, 2008.
- ↑ IMPROVE-IT study confirms low LDL cholesterol as a key factor in preventing cardiovascular events
- ↑ Ezetimibe: First evidence of cardiovascular protection through controversial lipid-lowering drugs