Ciclosporin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
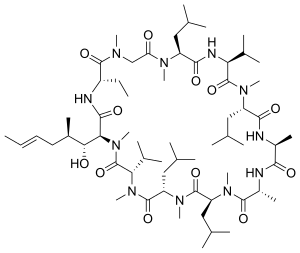 
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Ciclosporin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 62 H 111 N 11 O 12 | |||||||||||||||||||||
| Brief description |
white solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
Calcineurin inhibitor |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 1202.61 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
148-151 ° C |
|||||||||||||||||||||
| solubility |
soluble in methanol , ethanol, chloroform and ether |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Ciclosporin ( INN ), also called Cyclosporin A , is a drug from the group of immunosuppressants , which is isolated from the Norwegian sac fungi Tolypocladium inflatum ( W. Gams ) and Cylindrocarpon lucidum (Booth). The substance is a cyclic peptide that consists of eleven amino acids .
Ciclosporin suppresses the immune defense by indirectly inhibiting the enzyme calcineurin , and is mainly used in transplant medicine to prevent rejection reactions. Ciclosporin has a narrow therapeutic range , so that regular blood level controls (therapeutic drug monitoring ) are necessary when used internally .
Jean-François Borel and Hartmann Stähelin at Sandoz in Basel are considered to be the discoverers in the early 1970s. In a transplant, cyclosporine was first used in 1978 by Roy Calne at Cambridge University . Its use revolutionized this branch of medicine , and patient survival times increased significantly.
pharmacology
Mechanism of action
Cyclosporin binds to the immunophilin cyclophilin A (a prolyl-cis-trans isomerase ) and this complex binds to the calcineurin (a calcium / calmodulin-dependent phosphatase). Thus, the binding to NF-AT (abbreviation for nuclear factor activating T-Cell ), a gene-regulating protein , is blocked in the cell plasma . In activated T cells , calcineurin is responsible for the dephosphorylation of NF-AT. NF-AT can then be translocated into the cell nucleus , where it can activate the reading of genes for numerous cytokines and cell surface receptors (including interleukin-2 and γ-interferon ). The calcineurin inhibition therefore inhibits the release of immune-stimulating substances and thus the activation and reproduction of lymphocytes . In this way, it acts as an immunosuppressant.
application areas
Medicines containing ciclosporin are used for ulcerative colitis , Crohn's disease , glomerulonephritis , skin diseases (especially severe, therapy-resistant forms of atopic dermatitis and psoriasis) and chronic inflammatory conditions of the conjunctiva and cornea (ciclosporin-A eye drops according to NRF 15.21). It is also used against alopecia areata , in combination with methylprednisolone with very high treatment success. In veterinary medicine, it is occasionally used for atopic dermatitis in dogs and chronic stomatitis in cats.
In the treatment of tumors , together with verapamil , it prevents the transport of certain tumor chemotherapeutic agents out of the cell through the multidrug resistance glycoprotein P-170 .
Cyclosporin A is also given after transplants to avoid rejection reactions. The discovery of this effect, after which Roy Calne first used cyclosporine as an immunosuppressant in a transplant in 1978 , effectively reduced defensive reactions and the number of heart transplants has increased steadily since 1981. Norman Shumway at Stanford University achieved the first long-term results with him.
Side effects
Possible side effects include damage to the kidneys , liver, and gastrointestinal tract . Ciclosporin can also lead to gum overgrowth , hirsutism , edema and high blood pressure .
Regular use of high doses of cyclosporine, as is the case with transplant patients , leads to a weakened immune system. This significantly increases the risk of cancer . Compared to the normal population, the probability of developing cancer is three to five times higher. In addition, there is an increased risk of infections - also due to immunosuppression. The blood lipid levels may be increased. Strong sunlight should be avoided when ingestion. For example, simultaneous light therapy (UV radiation) is ruled out when taken due to a skin disease . Ciclosporin can lead to fibroadenomas .
Trade names
Cicloral (D, A), Immunosporin (D), Neoimmun (A), Sandimmun Optoral (D, A), Sandimmun Neoral (CH), Ikervis (D) various generics (D), specialty: Restasis (USA; 0.05 %, aqueous suspension of cyclosporin A solution for the treatment of keratoconjunctivitis sicca , also approved in Germany under the name "Ikervis" since 2015), Verkazia
Atopica, Cyclavance, Sporimune, Optimmune
Web links
Individual evidence
- ↑ a b c Datasheet Cyclosporin A from Sigma-Aldrich , accessed on March 22, 2011 ( PDF ).
- ↑ Data sheet Cyclosporin A, Tolypocladium inflatum (PDF) from Calbiochem, accessed on December 7, 2015.
- ↑ mpbio.com: Cyclosporin A ( Memento of the original from December 18, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed January 1, 2016
- ^ Q. Huai, HY Kim et al. a .: Crystal structure of calcineurin-cyclophilin-cyclosporin shows common but distinct recognition of immunophilin-drug complexes. In: Proceedings of the National Academy of Sciences . Volume 99, Number 19, September 2002, pp. 12037-12042, doi : 10.1073 / pnas.192206699 . PMID 12218175 . PMC 129394 (free full text).
- ↑ New recipe form, recipe information for ciclosporin
- ↑ BJKim et al. (2008). Combination therapy of cyclosporine and methylprednisolone on severe alopecia areata . In Journal of Dermatological Treatment Volume 19, Issue 4, pp. 216-220. PMID 18608727
- ^ H. Wolff: On the development of surgery and surgical research in the GDR . German Society for Surgery - Announcements 1/2012, pp. 1–8
- ↑ J. Dantal, E. Pohanka: Malignancies in renal transplantation: an unmet medical need. In: Nephrology Dialysis Transplantation . 2007, Volume 22 Suppl 1: pp. I4-10. doi : 10.1093 / ndt / gfm085 . PMID 17456618 . (Review).
- ↑ Red List online, as of September 2009.
- ↑ AM comp. d. Switzerland, as of September 2009.
- ↑ AGES-PharmMed, as of September 2009.
- ↑ Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 17-20 July 2017 , EMA PM of July 21, 2017, accessed on July 28, 2017