Peptidyl-prolyl-cis-trans isomerases
| Peptidyl-prolyl-cis-trans isomerases | ||
|---|---|---|
|
||
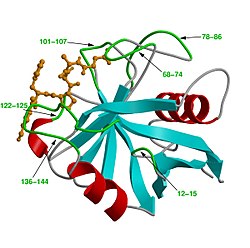
| Ribbon model of cyclophilin A (human) with peptide substrate (orange), according to PDB 1RMH | ||
| Enzyme classification | ||
| EC, category | 5.2.1.8 , isomerase | |
| Response type | Rotation of an amide bond | |
| Substrate | Peptidylproline (Ω = 180 °) | |
| Products | Peptidylproline (Ω = 0 °) | |
| Occurrence | ||
| Parent taxon | Creature | |
Peptidyl-prolyl-cis-trans-isomerases (also PPIases or PPI) are enzymes that occur in all living things. They catalyze the bond axis rotation of amide bonds in proteins in which the amino acid proline is involved. This reaction is the rate-limiting step in protein folding . At least 33 different PPIases are known in humans, several of which are already the target of active pharmaceutical ingredients.
PPIases are divided into three classes: cyclophilins , FK506-binding proteins, and parvulins .
Protein folding
The planarity of the peptide bond usually forces the ω angle to 180 ° (the common trans conformation ) or 0 ° (the rare cis conformation ). The cis conformation is mainly observed when proline is involved in a peptide bond. This bond can exist in either the trans or the cis configuration; a change between the two configurations takes place only slowly. This is related to the cyclic structure of the proline molecule, in which the amino group is connected to the central carbon atom (α-carbon atom) and the side chain of the amino acid, which is not the case with any other proteinogenic amino acid.
The specific protein folding (formation of the three-dimensional structure) of such proteins, which among other things depends on the spatial structures of the individual bonds, cannot therefore take place spontaneously. The peptidyl-prolyl-cis-trans-isomerases act here as folding helpers . The enzymes are able to switch between the cis and the trans configuration of the respective peptide bond so that the protein folds correctly and only then can fulfill its function. Both cis and trans configurations can arise.
Individual evidence
- ↑ Search result EC: 5.2.1.8 at UniProt .
- ↑ Search result EC: 5.2.1.8 (human) at UniProt .
- ↑ Natalya K. Nagradova: Foldases: enzymes catalyzing protein folding . Nova Biomedical Books, New York 2008, ISBN 1-60456-389-3 , pp. 51 ff .
- ^ Arthur Greenberg, Curt M. Breneman, Jeffrey M. Liebman: The amide linkage: structural significance in chemistry, biochemistry and materials science . Wiley, New York 2003, ISBN 0-471-42025-5 , pp. 75 ff .
