Peptide bond
A peptide bond is a carboxylic acid amide - bond between the carboxy group of an amino acid and the amino group of the α-carbon atom (α-C-atom) of a second amino acid.
Formally, for example, two molecules of the can proteinogenic amino acid alanine in a condensation reaction with elimination of water to the dipeptide alanyl-alanine react ( condense ):
Since the amino group is too weakly nucleophilic to react directly with the carboxy group, or can also be present in protonated form as -NH 3 (+) , the equilibrium is on the left under normal conditions . The chemical reaction is endergonic .
Both in peptide synthesis in the laboratory and in the biological synthesis of peptides and proteins , the reactive groups must first be activated. In biological systems, this is mostly done by enzymes . In protein biosynthesis in a cell , this reaction is catalyzed by the ribosomes during translation . In addition, non-ribosomal peptide synthetases (NRPS) also occur in some organisms as enzymes that enable non-ribosomal peptide synthesis .
Additional amino acids (AA) can be linked via peptide bonds through multiple condensation. Dipeptides (2 AA) then result in tripeptides (3), tetrapeptides (4), pentapeptides (5), hexapeptides (6), heptapeptides (7), octapeptides (8), nonapeptides (9), etc., with such peptides from a few amino acids, called oligopeptides , can be distinguished from even larger peptides made up of many amino acids, called polypeptides . The chain-like polypeptides made up of numerous amino acids belong to the macromolecules .
Polypeptide chains from different amino acids form the primary structural element of proteins and are characterized by their amino acid sequence . For information on primary, secondary and tertiary structures of peptides and proteins, see protein structure .
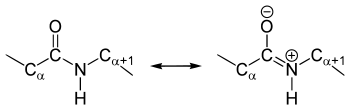
The crystal structure analyzes of amino acids and dipeptides show that the amide group is planar, i.e. all atoms involved in the structure are in one plane. The dihedral angle (HNCO) is 180 ° and the atoms can not be rotated against each other due to mesomerism stabilization - the peptide bond is therefore only rotationally flexible to a limited extent. This restricted rotation can be shown in a Ramachandran plot or a Janin plot .
In the native proteins there are predominantly trans peptide bonds, cis peptide bonds are mainly found in cyclic dipeptides ( diketopiperazines ) and cyclic tripeptides (example: cyclotriprolyl). The reason for the frequent occurrence of trans -Peptidbindungen are the steric hindrance between the groups at the α-C-atom in cis -Peptidbindungen that in the trans - configuration not occur.
The bond lengths are between nitrogen and carbonyl - carbon 133 pm , between nitrogen and α-carbon atom 146 pm, between carbonyl carbon and α-carbon atom 151 pm and between carbonyl carbon and oxygen 124 pm. The shorter length of the CN bond in the amide bond compared to the normal CN bond indicates that it has the character of a double bond ; this type of chemical bond is also called a partial double bond. This peculiarity is explained by the amide-iminol tautomerism of the peptide bond.
Peptides and amides

The reaction of the carboxy group of one amino acid and the amino group of a second amino acid forms a peptide bond with elimination of water. Every peptide bond is also an amide bond .
A prerequisite for the formation of a peptide bond is the condensation reaction of the terminal carboxy group on the C 1 atom with the amino group on the α-C atom of a second amino acid. Any other condensation between carboxy group and amino group also leads to an amide bond, which is not, however, a peptide bond.
If peptides are lengthened by means of peptide bonds, the terminal carboxy or amino groups on the α-C atom react with further amino acids.
Individual evidence
- ↑ Hans-Dieter Jakubke, Hans Jeschkeit: Amino acids, peptides, proteins. Verlag Chemie, Weinheim 1982, ISBN 3-527-25892-2 .
- ↑ Jeremy M. Berg, Lubert Stryer, John L. Tymoczko: Stryer Biochemistry . Springer-Verlag, Berlin, Heidelberg 2015, ISBN 978-3-8274-2989-6 , pp. 37 ( limited preview in Google Book search).
- ^ A b Hans-Dieter Jakubke, Hans Jeschkeit: Amino acids, peptides, proteins. Verlag Chemie, Weinheim 1982, ISBN 3-527-25892-2 , pp. 96-97.



