Hexapeptides
Hexapeptides are oligopeptides made up of six amino acid building blocks. The term "amino acid building block" comes from the fact that the individual amino acids are linked to one another via peptide bonds . Such a bond is formed by splitting off water between the amino group of one amino acid and the carboxy group of another amino acid.
Like most peptides , the majority of hexapeptides also take on an important role in the organism of a living being and / or are pharmacologically active.
Linear hexapeptides
Linear hexapeptides are understood as meaning hexapeptides in which the amino acid building blocks are linked to one another in a chain via five peptide bonds.
Argireline
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
|
|
Argireline is also called acetylhexapeptide-8 or acetylhexapeptide-3. It is a synthetic hexapeptide with an acetyl group at the N terminus and an amino group at the C terminus, which was produced in 2002 by a group led by the Spanish chemist Antonio Ferrer-Montiel (* 1962). This peptide inhibits neurotransmitter release , just like botox . For this reason, it is used to reduce expression lines. To do this, Argireline is applied to the skin via a cream that is non-invasive and non-toxic. The advantage over Botox is that Argireline is suitable for those who suffer from neuromuscular or autoimmune diseases or who are botox intolerant. However, the effect of Argireline is not comparable to that of Botox.
Calpinactam
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
Calpinactam comes in the fungus Mortierella alpina ago |
Calpinactam was isolated from the fungus Mortierella alpina in 2009 by a group led by the Japanese Hiroshi Tomoda (* 1955) . What is special about the structure of this hexapeptide is that the C terminus is formed by α-amino- ε-caprolactam , which can be obtained from lysine .
The peptide is effective against the bacterial species Mycobacterium smegmatis and Mycobacterium tuberculosis .
GHRP-6
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
|
|
GHRP-6 is the English abbreviation for G rowth H ormone- R eleasing P eptide-6 and means “growth hormone releasing hexapeptide”. It is therefore a growth hormone secretagogue and also improves the tissue viability of various organs. GHRP-6 is a synthetic peptide that was first produced in 1983 by a group led by the American Cyril Y. Bowers.
Neuromedin N
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
Neuromedin N is found in the spinal cord of pigs |
Neuromedin N is a neuropeptide with an effect similar to neurotensin . In 1984 a group led by the Japanese Naoto Minamino (* 1953) isolated it from the spinal cord of pigs for the first time.
Cyclic hexapeptides
Cyclic hexapeptides are hexapeptides with a circular shape . In contrast to the linear hexapeptides, the formation of a sixth peptide bond leads to a ring closure . This can also take place if there is another covalent bond .
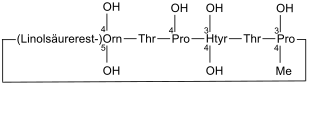
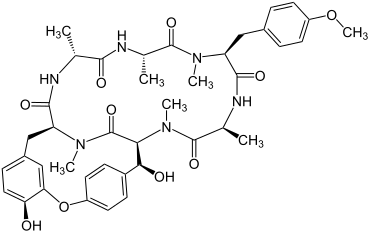
Echinocandin B
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
 Echinocandin B comes in the mold Aspergillus nidulans ago |
Echinocandin B is an antifungal drug . It was isolated from the Aspergillus nidulans mold in 1974 by the Swiss company CIBA-GEIGY . This peptide inhibits the synthesis of β- (1,3) - D -glucan, an important component of the cell walls of fungi, so that it has an antifungal effect (e.g. against Candida albicans ).
What is special about the structure of Echinocandin B is that this peptide contains linoleic acid , which is said to lead to hemolysis . In order to check whether there are other peptides that also prevent the synthesis of β- (1,3) - D -glucan and are more efficient, further naturally occurring peptides were searched for and analogues of these were produced. This includes B. Caspofungin .
Bouvardin
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
Bouvardin is in the plant Bouvardia ternifolia (Rubicae) contain |
Bouvardin was isolated from the Bouvardia ternifolia (Rubicae) plant in 1977 by a research group led by the American Jack R. Cole (* 1929) . This peptide shows anti-tumor activity against lymphatic leukemia and against malignant melanoma , in particular by inhibiting protein synthesis.
See also
literature
- E. Boschetti, P. Giorgio Righetti: Hexapeptide combinatorial ligand libraries: the march for the detection of the low-abundance proteome continues. In: BioTechniques. Volume 44, Number 5, April 2008, pp. 663-665, doi: 10.2144 / 000112762 . PMID 18474042 .
- A. Fröhlich, C. Lindermayr: Deep insights into the plant proteome by pretreatment with combinatorial hexapeptide ligand libraries. In: Journal of Proteomics. Volume 74, number 8, August 2011, pp. 1182–1189, doi: 10.1016 / j.jprot.2011.02.019 . PMID 21354349 .
Individual evidence
- ↑ a b c d e f G. D. Fassman (Ed.): Handbook of Biochemistry an Molecular Biology. Part A: Protein. Volume I, 3rd Edition, CRC Press, Cleveland 1976, ISBN 0-87819-504-1 , pp. 1-108.
- ↑ F. Gorouhi, HI Maibach: Review Article - Role of topical peptides in preventing or treatingaged skin. In: International Journal of Cosmetic Science . Volume 31, 2009, pp. 327-345, doi: 10.1111 / j.1468-2494.2009.00490.x .
- ↑ J. Villalaín Boullon: Translational Biophysics - A conversation with Antonio Ferrer-Montiel. In: Biofisica Magazine . Volume 6, 2016, pp. 11–15, online version , accessed on July 13, 2017.
- ↑ a b C. Blanes-Mira, J. Clementy, G. Jodasy, A. Gil, G. Fernandez-Ballester, B. Ponsatiy, L. Gutierrez, E. Perez-Paya, A. Ferrer-Montie: A synthetic hexapeptide (Argireline) with anti-wrinkle activity. In: International Journal of Cosmetic Science . Volume 24, 2002, pp. 303-310, doi: 10.1046 / j.1467-2494.2002.00153.x .
- ↑ Raja K Sivamani: Cosmeceuticals and Active Cosmetics. 3. Edition. CRC Press, 2016, ISBN 978-1-4987-8246-3 .
- ↑ Big promises, but few facts about the active ingredient Argireline: The “gentle alternative” to Botox does not yet exist. at: nzz.ch , accessed on May 17, 2017.
- ↑ a b c d N. Koyama, S. Kojima, T. Fukuda, T. Nagamitsu, T. Yasuhara, S. Omura, H. Tomoda: Structure and Total Synthesis of Fungal Calpinactam, A New Antimycobacterial Agent. In: Organic Letters . Volume 12, No. 3, 2010, pp. 432-435, doi: 10.1021 / ol902553z .
- ↑ Prabook: Profile of Hiroshi Tomoda. Retrieved on: July 13, 2017.
- ↑ N. Koyama, S. Kojima, K. Nonaka, R. Masuma, M. Matsumoto, S. Omura, H. Tomoda: Calpinactam, a new anti-mycobacterial agent, produced by Mortierella alpina FKI-4905. In: The Journal of Antibiotics . Volume 63, 2010, pp. 183-186, doi: 10.1038 / ja.2010.14 .
- ↑ A. Cabralesa, J. Gilb, E. Fernándezc, C. Valenzuelad, F. Hernándezd, I. Garcíad, A. Hernández, V. Besadab, O. Reyesa, G. Padrónb, J. Berlangae, G. Guillénf, LJ González: Pharmacokinetic study of Growth Hormone-Releasing Peptide 6 (GHRP-6) in nine male healthy volunteers. In: European Journal of Pharmaceutical Sciences . Volume 48, No. 1–2, pp. 40–46, doi: 10.1016 / j.ejps.2012.10.006 .
- ↑ FA Momany, CY Bowers, GA Reynolds, A. Hong, K. Newlander: Conformational Energy Studies and in Vitro and in Vivo Activity Data on Growth Hormone-Releasing Peptides. In: Endocrinology . Volume 114, No. 5, 1984, pp. 1531-1536, doi: 10.1210 / endo-114-5-1531 .
- ↑ a b N. Minamino, K. Kangawa, H. Matsuo: Neuromedin N: A novel neurotensin-like peptide identified in porcine spinal cord. In: Biochemical and Biophysical Research Communications . Vol. 122, No. 2, 1984, pp. 542-549, doi: 10.1016 / S0006-291X (84) 80067-4 .
- ↑ J. Falbe, M. Regitz (Ed.): Römpp Lexikon Chemie. Georg Thieme Verlag, Stuttgart 1998, ISBN 3-13-734910-9 , p. 2876.
- ↑ The information on the year of birth comes from Naoto Minamino personally (e-mail contact on July 14, 2017)
- ↑ a b F. Benz, F. Knüsel, J. Nüesch, H. Treichler, W. Voser, R. Nyfeler, W. Keller-Schierlein: 267. Metabolic products of microorganisms - Part 143 - Echinocandin B, a novel polypeptide Antibiotic from Aspergillus nidulans var. Echinulatus : insulation and building blocks. In: Helvetica Chimica Acta . Volume 57, No. 8, 1974, pp. 2459-2477, doi: 10.1002 / hlca.19740570818 .
- ↑ MB Kurtz, CM Douglas: Lipopeptide inhibitors of fungal glucan synthase. In: Journal of Medical & Veterinary Mycology . Volume 35, No. 2, 1997, pp. 79-86, doi: 10.1080 / 02681219780000961 .
- ↑ LL Klein, L. Li: Design and Preparation of Cyclopeptamine Antifungal Agents. In: Current Pharmaceutical Design . Volume 5, No. 2, 1999, pp. 57-71, limited preview in Google Book Search.
- ↑ G. Erkel: Non-β-Lactam Antibiotics. In: K. Esser, M. Hofrichter (Ed.): The Mycota - Industrial Applications X. 2nd edition. Springer Verlag, Berlin / Heidelberg 2010, ISBN 978-3-642-11457-1 , pp. 125f. limited preview in Google Book search.
- ↑ a b c S. D. Jolad, JJ Hoffman, SJ Torrance, RM Wiedhopf, JR Cole, SK Arora, RB Bates, RL Gargiulo, GR Kriek: Bouvardin and Deoxybouvardin, Antitumor Cyclic Hexapeptides from Bouvardia ternifolia (Rubiaceae). In: Journal of the American Chemical Society . Volume 99, No. 24, 1977, pp. 8040-8044, doi: 10.1021 / ja00466a043 .
- ↑ Jacques Cattell Press (Ed.): 2. C – F. In: American Men & Women of Science. Physical and Biological Sciences. 16th edition. RR Bowker Company . New York 1986, ISBN 0-8352-2223-3 , p. 307.
- ↑ MP Chitnis, AD Alate, RS Menon: Effect of Bouvardin (NSC 259968) on the Growth Characteristics and Nucleic Acids and Protein Syntheses Profiles of P388 Leukemia Cells. In: Chemotherapy . Volume 27, No. 2, 1981, pp. 126-130, doi: 10.1159 / 000237967 .
- ^ RK Johnson, MP Chitnis: Antineoplastic and Biochemical Effects in Murine Tumors of Bouvardin, A Cyclic Hexapeptide from Bouvardia terniflora. In: Proceedings of the American Association for Cancer Research and American Society of Clinical Oncology . No. 19, 1978, p. 218.