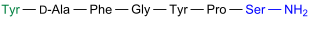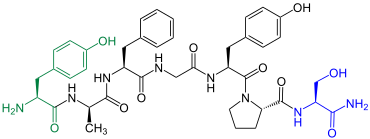Heptapeptides
Heptapeptides are oligopeptides made up of seven amino acid building blocks. The term "amino acid building block" comes from the fact that the individual amino acids are linked to one another via peptide bonds . Such a bond is formed by splitting off water between the amino group of one amino acid and the carboxy group of another amino acid.
Like most peptides , the majority of heptapeptides also take on an important role in the organism of a living being and / or are pharmacologically active.
Linear heptapeptides
Heptapeptides have a linear structure when the amino acid building blocks are linked in a chain via six peptide bonds.
β-casomorphine-7
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
β-casomorphin-7 is in the casein - peptone of bovine contain |
β-Casomorphin-7 was in 1979 by a group led by the German physician Hans Jörg Teschemacher (1938 *) from bovine casein - peptone isolated. This peptide is one of the opioid peptides and cannot be broken down by the pronase enzyme , which is a special feature.
Deltorphin-I and Deltorphin-II
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence | |
|
|
|
The Deltorphins are in the skin of the frog bicolor Phyllomedusa included |
Deltorphin-I contains the amino acid component of aspartic acid and Deltorphin-II that of glutamic acid . In 1989 they were extracted from the skin of the frog Phyllomedusa bicolor by a group led by the Italian pharmacologist Vittorio Erspamer (1909–1999) . They belong to the opioid peptides and bind selectively to the δ-opioid receptors .
Dermorphine
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
Dermorphin in the skin of the frog is Phyllomedusa sauvagei included |
Demorphin is one of the opioid peptides. It was first extracted from the skin of the frog Phyllomedusa sauvagei in 1980 by a group led by the Italian Pier Carlo Montecucchi .
In contrast to deltorphin-I and deltorphin-II , demorphin binds selectively to the μ-opioid receptors .
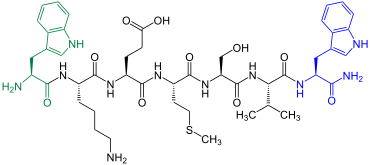
Wwamid-1
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
Wwamid-1 is in the ganglia of the African snail Achatina fulica contain |
Wwamid-1 was extracted from the ganglia of the African snail Achatina fulica in 1993 by a Japanese group led by Hiroyuki Minakata (* 1956) . This neuropeptide is an inhibitor of a central neuron in the snail. It also shows modeling effects in the peripheral nervous system of various molluscs , i.e. H. it inhibits or promotes muscle contractions. It is not yet clear what is happening. It could be important for controlling muscle contractions.
Cyclic heptapeptides
Cyclic heptapeptides are understood to mean heptapeptides with a circular shape . This shape occurs when a seventh peptide bond is formed. Such a ring closure is also possible through the formation of other covalent bonds. This is e.g. B. the case with Viroisin.
Phalloidin
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
Phalloidin occurs in the fungus Amanita phalloides , the green capillary mushroom |
Phalloidin is a bicyclic heptapeptide and belongs to the phallotoxins . This is a group of toxins , which in green death cap ( Amanita phalloides were found). In 1937 the two Germans Feodor Lynen (1911–1979) and Ulrich Wieland (1911–2003) succeeded in isolating the toxic peptide in crystalline form from this fungus.
It is characteristic of the mode of action of this heptapeptide that liver cell membranes are changed in such a way that protuberances form and blood is absorbed. This is due to the fact that phalloidin inhibits the depolymerization of filamentous F-actin to monomeric G-actins, i.e. the breakdown of microfilaments .
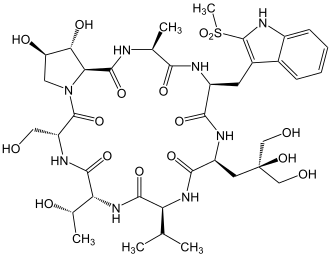
Viroisin
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
Viroisin comes in the fungus in Europe Amanita virosa ago |
Viroisin is one of the virotoxins . These are monocyclic, toxic heptapeptides found in the death cap mushrooms . In Europe, this group of toxic peptides appears to be found only in the Amanita virosa mushroom . In 1980 Heinz Faulstich and colleagues succeeded in isolating viroisin from this fungus.
Viroisin's mode of action is very similar to that of phalloidin . Like phalloidin, viroisin also prevents the depolymerization of filamentous F-actins , so that blood is also absorbed into the liver.
In the case of viroisin, it appears that this peptide is only present in the active conformation when it is bound to actin according to the induced fit mechanism .
See also
Individual evidence
- ↑ a b c d e f G. D. Fassman (Ed.): Handbook of Biochemistry an Molecular Biology. (= Proteins. Volume I). 3. Edition. CRC Press, Cleveland 1976, ISBN 0-87819-504-1 , pp. 1-108.
- ↑ a b c V. Brantl, H. Teschemacher, A. Henschen, F. Lottspeich: Novel Opioid Peptides Derived from Casein (β-Casomorphins). II. Structure of Active Components from Bovine Casein Peptone. In: Hoppe-Seyler's Journal for Physiological Chemistry. Volume 360, No. 9, 1979, pp. 1217-1224, doi: 10.1515 / bchm2.1979.360.2.1211 .
- ↑ Rudolf book Hein Department of Pharmacology: History of Rudolf-Buchheim Institute of Pharmacology - First of Pharmaceutics. September 27, 2007. Accessed: July 3, 2017.
- ↑ a b c V. Erspamer, P. Melchiorri, G. Falconieri-Erspamer, L. Negri, R. Corsi, C. Severini, D. Barra, M. Simmaco, G. Kreil: Deltorphins: A Family of Naturally Occurring Peptides With High Affinity and Selectivity for Delta Opioid Binding Sites. In: Proceedings of the National Academy of Sciences USA. Volume 86, No. 13, 1989, pp. 5188-5192, doi: 10.1073 / pnas.86.13.5188 .
- ^ A b P. C. Montecucchi, R. de Castiglione, S. Piani, L. Gozzini, V. Erspamer: Amino acid composition and sequence of dermorphin, a novel opiate-like peptide from the skin of Phylomedusa sauvagei. In: International Journal of Peptide and Protein Research. Volume 17, No. 3, 1981, pp. 275-283, doi: 10.1111 / j.1399-3011.1981.tb01993.x .
- ↑ a b c H. Minakata, T. Ikeda, Y. Muneoka, M. Kobayashi, K. Nomoto: WWamide-1, -2 and -3: novel neuromodulatory peptides isolated from ganglia of the African giant snail, Achatina fulica. In: FEBS letters. Volume 323, No. 1-2, 1993, pp. 104-108, doi: 10.1016 / 0014-5793 (93) 81458-C .
- ↑ The information on the year of birth comes from Hiroyuki Minakata personally (email contact on July 7, 2017).
- ↑ a b T. Wieland, HW Schnabel: About the toxins of the green leaf mushroom - New sequence analysis of phalloidin and phalloin. In: Justus Liebig's Annals of Chemistry. Volume 657, No. 1, 1962, pp. 225-228, doi: 10.1002 / jlac.19626570128 .
- ↑ R. Hansel, E. Steinegger: Pharmakognosie und Phytopharmazie. 4th edition. Springer-Verlag, Berlin / Heidelberg 1988, ISBN 3-662-08319-1 , p. 475, limited preview in the Google book search.
- ^ Association for computer genealogy: Gravestone of the Wieland family. 2013, Retrieved on: July 6, 2017.
- ↑ F. Lynen, U. Wieland: About the toxins of the death cap mushroom. VI. In: Justus Liebig's Annals of Chemistry . Volume 533, No. 1, 1937, pp. 93-117, doi: 10.1002 / jlac.19385330105 .
- ^ A. Bresinsky, H. Besl: Poison mushrooms - A manual for pharmacists, doctors and biologists. Wissenschaftliche Verlagsgesellschaft, Stuttgart 1985, ISBN 3-8047-0680-0 , pp. 23-24.
- ↑ T. Wieland: Amatoxins, Phallotoxins - the poisons of the death cap mushroom. In: Chemistry in Our Time . Volume 13, No. 2, 1979, pp. 56-63, doi: 10.1002 / ciuz.19790130205
- ^ J. Rassow, K. Hauser, R. Netzker, R. Deutzmann: Biochemistry. 3. Edition. Georg Thieme Verlag, Stuttgart 2012, ISBN 978-3-13-125353-8 , p. 376ff.
- ↑ a b c d e H. Faulstich, A. Buku, H. Bodenmüller, T. Wieland: Virotoxins: actin-binding cyclic peptides of Amanita virosa mushrooms. In: Biochemistry. Volume 19, No. 14, 1980, pp. 3334-3343, doi: 10.1021 / bi00555a036 .
- ↑ a b A. Bresinsky, H. Besl: Giftpilze – A manual for pharmacists, doctors and biologists. Wissenschaftliche Verlagsgesellschaft, Stuttgart 1985, ISBN 3-8047-0680-0 , pp. 19-21.