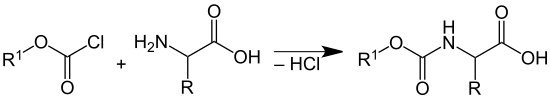Peptide synthesis
The peptide synthesis treated process for the preparation of biochemically important class of substances of the peptides .
description
Defined peptides cannot be produced by direct condensation of amino acids . The chemical reaction of two different unmodified amino acids can produce four different dipeptides . For a targeted synthesis of further defined peptides, both the amino group of one amino acid and the carboxy group of the other amino acid must therefore be temporarily blocked by a suitable protective group . In addition, activation of the carboxy group is necessary, which is to react with the amino group of the second amino acid to form a peptide bond , since carboxylic acids usually only react with amines to form salts and do not form a covalent bond . The formation of the peptide bond must take place under conditions in which no racemization can occur if enantiomerically pure products are to arise. With the exception of achiral glycine , ribosomally produced peptides and proteins consist exclusively of L- amino acids. To protect the carboxy group, for example, the first amino acid can be reacted with isobutene in the presence of sulfuric acid to form the amino acid tert-butyl ester:
In some cases, such as in asparagine , this method fails and the tert -butyl ester can only by transesterification with acetic acid tert -butyl ester are obtained in the presence of strong acids.
To protect the amino group of the second amino acid, the amino group is, for example, with benzyl chloroformate (abbreviation: Z-Cl or Cbz-Cl; R 1 = benzyl group), fluorenylmethyloxycarbonyl chloride (abbreviation: Fmoc-Cl; R 1 = fluorenyl-9-methyl group) or with di - tert -butyl dicarbonate (BOC anhydride) converted to the corresponding urethanes :
After the formation of the peptide bond can be of the tert -butyl esters with acids such as trifluoroacetic acid , with elimination of tert -butanol hydrolyze , wherein the carboxylic acid is formed again. The urethanes are split either by reaction with hydrogen chloride / glacial acetic acid (splitting of the tert-butyl urethane) by a solution of piperidine (splitting of fluorenyloxycarbonyl) or by hydrogenolysis (splitting of the benzyl urethane), in each case with the release of the amino function.
The carboxy group is activated, for example, by converting it into a carboxylic acid chloride , a carboxylic acid azide or a 4-nitrophenyl ester. The activation of the carboxy group takes place particularly frequently through the use of dicyclohexylcarbodiimide (DCC) as a coupling reagent . Active esters of N -hydroxybenzotriazole (HOBt), 1-hydroxy-7-azabenzotriazole or N -hydroxysuccinimide (HOSu), which are either mixed with the condensation agent N , N -diisopropylcarbodiimide (DIC), with 3-ethyl-1 ( N , N -dimethyl) aminopropylcarbodiimide (EDCI) or with HATU , HCTU , TBTU , ethyl-2-cyano-2- (hydroxyimino) acetate ( Oxyma ), COMU , TOMBU or COMBU .
An elegant method for peptide synthesis is the solid phase synthesis according to Merrifield . In peptide synthesis, the peptides with uncoupled after each synthesis cycle acetic anhydride acetylated ( English capping , bekappen '), that they also do not couple in cycles, because they would otherwise exposed to one cycle and would be flawed. Polystyrene (PS), polyamide , polyethylene glycol (PEG) or PEG-modified PS are usually used as the solid phase .
The products of a peptide synthesis are usually then purified , e.g. B. by precipitation or by HPLC . By using a further polymerizable molecule in the last coupling step of the peptide synthesis, the correctly synthesized peptides can be separated from the defective and acetylated coupling products after a subsequent polymerization.
Since the yield decreases with each coupled amino acid, proteins with a length of more than 70 amino acids are often produced by protein ligation from two or more peptides.
Alternatives
An alternative method for peptide synthesis is the Bailey peptide synthesis . It is based on the use of NCAs as starting materials. An activated carboxy group can also be represented using the N -carboxylic acid anhydride method in the form of α- amino acid N -carboxy anhydrides (NCAs or Leuchs' anhydrides ).
use
Synthetic peptides serve as inhibitors or substrates of enzymes or serve as antigens in immunology .
history
The first peptide synthesis was carried out by the Nobel Prize winner Emil Fischer , who produced around 100 peptides from 1902 to 1907, the longest of which consisted of 17 amino acids. In later years his student Emil Abderhalden significantly improved peptide synthesis. The first hormonal peptide produced was oxytocin (a cyclic nonapeptide ) synthesized by Vincent du Vigneaud in 1954 . In 1955 he received the Nobel Prize in Chemistry for his work on the biochemically important sulfur compounds, in particular for the first synthesis of a polypeptide hormone (oxytocin) . The first syntheses of insulin took place in the years 1963 to 1966 by Helmut Zahn , Y. Wang and Panayotis Katsoyannis .
The first solid phase synthesis of peptides on solid polymers as substrates was carried out in 1966 by Bruce Merrifield and Arnold Marglin with the Merrifield synthesis using the example of insulin. As a result, the yield per reaction cycle rose from 90% to 99.5% and the secondary reaction products and starting materials could be easily removed by washing, whereby longer peptides could also be produced with acceptable yield. In 1984 he received the Nobel Prize in Chemistry for his development of the methodology for chemical synthesis on a solid matrix .
literature
- WC Chan, PD White: Fmoc Solid Phase Peptide Synthesis. Reprint 2004, Oxford University Press, ISBN 0-19-963724-5 .
Web links
- Peptide Synthesis (PDF; 2.1 MB) University of Marburg, accessed February 4, 2011
Individual evidence
- ^ Siegfried Hauptmann : Organic chemistry . Harri Deutsch, 1985, ISBN 3-87144-902-4 , p. 661.
- ↑ Emil Taschner, Andrzej Chimiak, Jan F. Biernat, Czeslaw Wasielewski, Teresa Sokolowska: New esterification in peptide chemistry, IX. Representation of the ω-tert-butyl esters of carbobenzoxy-glutamic acid and -aspartic acid and their use for syntheses of α-dipeptides : In Justus Liebigs Annalen der Chemie , 1963, 663, 188-193 doi : 10.1002 / jlac.19636630127 .
- ^ Hans Beyer and Wolfgang Walter : Organic Chemistry . S. Hirzel, Stuttgart, 1984, ISBN 3-7776-0406-2 , pp. 791-794.
- ↑ CU Hjørringgaard, A. Brust, PF Alewood: Evaluation of COMU as a coupling reagent for in situ neutralization Boc solid phase peptide synthesis. In: Journal of peptide science: an official publication of the European Peptide Society. Volume 18, Number 3, March 2012, pp. 199-207, doi : 10.1002 / psc.1438 , PMID 22252935 .
- ↑ R. Subirós-Funosas, R. Prohens, R. Barba, A. El-Faham, F. Albericio: Oxyma: an efficient additive for peptide synthesis to replace the benzotriazoles-based HOBt and HOAt with a lower risk of explosion. In: Chemistry. Volume 15, number 37, September 2009, pp. 9394-9403, doi : 10.1002 / chem.200900614 . PMID 19575348 .
- ↑ R. Subirós-Funosas, L. Nieto-Rodriguez, KJ Jensen, F. Albericio: COMU: scope and limitations of the latest innovation in peptide acyl transfer reagents. In: Journal of peptide science: an official publication of the European Peptide Society. Volume 19, Number 7, July 2013, ISSN 1099-1387 , pp. 408-414, doi : 10.1002 / psc.2517 , PMID 23712932 .
- ↑ YE Jad, SN Khattab, BG de la Torre, T. Govender, HG Kruger, A. El-Faham, F. Albericio: TOMBU and COMBU as Novel Uronium-type peptide coupling reagents derived from Oxyma-B. In: Molecules. Volume 19, number 11, 2014, pp. 18953-18965, doi : 10.3390 / molecules191118953 , PMID 25412042 .
- ↑ A. El-Faham, F. Albericio: Peptide coupling reagents, morethan a letter soup. In: Chemical Reviews . Volume 111, Number 11, November 2011, pp. 6557-6602, doi : 10.1021 / cr100048w , PMID 21866984 .
- ↑ M. Zhang, D. Pokharel, S. Fang: Purification of Synthetic Peptides Using a Catching Full-Length Sequence by Polymerization Approach. In: Organic letters. February 2014, doi : 10.1021 / ol403426u . PMID 24527740 .
- ^ Daniel Zerong Wang: Comprehensive Organic Name Reactions and Reagents . tape 1 . John Wiley & Sons, Inc., Hoboken New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 156-159 .
- ↑ Hans-Dieter Jakubke, Hans Jeschkeit: Amino acids, peptides, proteins. Verlag Chemie, Weinheim, pp. 164-166, 1982, ISBN 3-527-25892-2 .
- ↑ Hans Rytger Kricheldorf: α-Aminoacid-N-Carboxy-Anhydrides and Related Heterocycles. Syntheses, Properties, Peptide Synthesis, Polymerization. Springer Verlag, Berlin Heidelberg, 1987, pp. 1-4, doi: 10.1007 / 978-3-642-71586-0 , ISBN 978-3-642-71588-4 (print), ISBN 978-3-642-71586 -0 (online).
- ↑ Ber. d. Deut. Chem. Ges. 36 (1903), p. 3982.
- ↑ Ber. d. Deut. Chem. Ges. 37 (1904), p. 2486.
- ↑ Ber. d. Deut. Chem. Ges. 40 (1907), pp. 1755, 1764.
- ↑ Ber. d. Deut. Chem. Ges. 64 (1931), p. 2070.
- ↑ Vincent du Vigneaud, Charlotte Ressler, John M. Swan, Carleton W. Roberts, Panayotis G. Katsoyannis: The Synthesis of Oxytocin. In: Journal of the American Chemical Society. 76, 1954, p. 3115, doi : 10.1021 / ja01641a004 .
- ↑ Information from the Nobel Foundation on the 1955 award ceremony for Vincent du Vigneaud (English)
- ^ Arnold Marglin: Insulin and solid-phase synthesis, 1964-1970. In: Biopolymers. 90, 2008, p. 200, doi : 10.1002 / bip.20912 .
- ↑ A. Marglin, RB Merrifield: The synthesis of bovine insulin by the solid phase method. In: Journal of the American Chemical Society. Volume 88, Number 21, November 1966, pp. 5051-5052, PMID 5978833 .
- ↑ a b Information from the Nobel Foundation on the 1984 award ceremony for Robert Bruce Merrifield (English)