N -carboxylic anhydride method
|
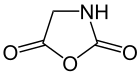
Oxazolidine-2,5-dione, |
The N -carboxylic acid anhydride method (also NCA method or Leuchs method) is a synthesis of organic chemistry . Hermann Leuchs (1879–1945) first described the method in 1906 as the intramolecular formation of α- amino acid N -carboxyanhydrides (NCAs or Leuchs' anhydrides ) starting from N -alkoxycarbonylamino acid chlorides.
Overview reaction
Leuchs originally converted N- alkoxycarbonylamino acids, such as glycine carboxylic acid, with thionyl chloride to give the respective acid chlorides. The gaseous by-products formed in this preparation of acid chlorides offer the advantage that the product does not need to be worked up. For example, the distillation of the amino acid chloride would already lead to the decomposition of the product. Leuch's methodology for the production of NCAs describes the subsequent intramolecular cyclization of the amino acid chloride with slight heating in a vacuum and elimination of an alkyl chloride . In the following, the Leuchs method is exemplified using the synthesis of oxazolidine-2,5-dione:
Newer sources also report on the synthesis of NCAs starting from phosgene , diphosgene , triphosgene , phosphorus tribromide or phosphorus pentachloride instead of thionyl chloride. The use of these reagents makes it possible, for example, to carry out the reaction with amino acids without protecting groups at higher temperatures. The following shows the synthesis of NCAs via the reaction of N -benzyloxycarbonylamino acids with phosgene in triethylamine (where R can be a hydrogen atom or an organic radical):
Reaction mechanism
The reaction mechanism is not mentioned in the literature and is therefore not discussed further at this point.
Atomic Economy and Use of NCAs
The preparation of the amino acids, for example the corresponding chlorides, leads to the formation of by-products such as acid waste, and the use of reagents is also required. The atom efficiency is already not optimal in the context of the preparation of the starting materials. The possible formation of halogenated by-products also ensures that there is no absolute atomic efficiency .
In spite of the suboptimal atomic efficiency, reactive products that are characterized by an activated carboxy group and can therefore be used in peptide syntheses are synthesized in this method . In 1949 J. Leggett Bailey developed a peptide synthesis based on the use of NCAs.
Individual evidence
- ^ A b Hans-Dieter Jakubke, Hans Jeschkeit: Amino acids, peptides, proteins. Verlag Chemie, Weinheim, pp. 164-166, 1982, ISBN 3-527-25892-2 .
- ↑ a b c d e f g Hans Rytger Kricheldorf: α-Aminoacid-N-Carboxy-Anhydrides and Related Heterocycles. Syntheses, Properties, Peptide Synthesis, Polymerization. Springer Verlag, Berlin Heidelberg, 1987, pp. 1-4, DOI: 10.1007 / 978-3-642-71586-0 , ISBN 978-3-642-71588-4 (print), ISBN 978-3-642-71586 -0 (online).
- ^ Fritz Kröhnke: Hermann Leuchs. 1879-1945. In: Chemical Reports. Volume 85, No. 11, 1952, pp. 55-89, DOI: 10.1002 / cber.19520851102 .
- ↑ a b c Hermann Leuchs: About the glycine carboxylic acid. In: Reports of the German Chemical Society. Volume 39, No. 1, 1906, pp. 857-861, DOI: 10.1002 / cber.190603901133 .
- ↑ a b Renée Wilder, Shahriar Mobashery: The Use of Triphosgene in Preparation of N-Carboxy-α-amino Acid Anhydrides. In: Journal of Organic Chemistry. Volume 57, No. 9, 1992, pp. 2755-2756, DOI: 10.1021 / jo00035a044 .
- ↑ J. Leggett Bailey: A new Peptide Synthesis. In: Nature. Volume 164, No. 4177, 1949, p. 889, DOI: 10.1038 / 164889a0 .