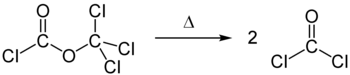Diphosgene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Crystal system |
monoclinic |
|||||||||||||||
| Space group |
P 2 1 / n (No. 14, position 2) |
|||||||||||||||
| Lattice parameters |
a = 5.5578 (5) Å, b = 14.2895 (12) Å, c = 8.6246 (7) Å, β = 102.443 (2) °, Z = 4 |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Diphosgene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 Cl 4 O 2 | |||||||||||||||
| Brief description |
colorless, pungent smelling liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 197.85 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.65 g cm −3 (14 ° C) |
|||||||||||||||
| Melting point |
−57 ° C |
|||||||||||||||
| boiling point |
128 ° C |
|||||||||||||||
| Vapor pressure |
13.33 h Pa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4584 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Diphosgene is a toxic chemical compound containing chlorine . Like phosgene, it was used as a lung warfare agent in World War I. It is also referred to as Pstoff .
history
Diphosgen was used for the first time on June 23, 1916 by German troops near Verdun in the area of Fort de Souville and Fort de Tavannes on the western front as a green cross warfare agent in garnet fillings.
Extraction and presentation
Technical manufacturing
Disphosgene is mostly produced industrially by photochlorination of methyl formate under UV light .
High-pressure mercury lamps are often used to generate UV light and the reaction is carried out in stirred tank reactors .
Laboratory scale
Because of the high volatility of methyl formate and its high, sometimes explosive reactivity, radical chlorination of methyl chloroformate is preferred , at least in the laboratory . This is available inexpensively and is obtained from phosgene and methanol :
Chemical properties
Diphosgene decomposes into two molecules of phosgene when heated ( thermolysis ).
use
Diphosgene is used as a less dangerous substitute for phosgene e.g. B. used in the production of carbonates , isocyanates and isocyanides . Militarily it was used as a weapon under the name Grünkreuz .
It also serves as a synthesis equivalent for phosgene ("dimeric phosgene") in the laboratory; in practice, however , the triphosgene is easier to handle.
Biological importance
Symptoms of poisoning with phosgene or diphosgene are excruciating coughing hours after inhalation, brownish sputum due to the admixture of blood, bluish skin ( cyanosis ) and pulmonary edema . If left untreated, phosgene or diphosgene poisoning ends in excruciating asphyxiation.
safety instructions
Diphosgene is highly toxic. The lethal dose LD 100 is 6 mg / l with an exposure time of one minute, the LC t50 3200 mg · min · m −3 and the IC t50 1600 mg · min · m −3 .
proof
The phosgene produced can be detected with test tubes.
See also
Individual evidence
- ^ A b c Valeria B. Arce, Carlos O. Della Védova, Anthony J. Downs, Simon Parsons, Rosana M. Romano: Trichloromethyl Chloroformate (“Diphosgene”), ClC (O) OCCl 3 : Structure and Conformational Properties in the Gaseous and Condensed Phases . In: Journal of organic chemistry . tape 71 , no. 9 , 2006, p. 3423–3428 , doi : 10.1021 / jo052260a (English).
- ↑ a b c d e f g Entry on trichloromethyl chloroformate in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c Entry on diphosgene. In: Römpp Online . Georg Thieme Verlag, accessed on September 2, 2014.
- ↑ Trichloromethyl chloroformate (diphosgene) . In: Carbonic acid derivatives (= methods of organic chemistry . Extension and follow-up volume to the 4th ed., Volume E4). Thieme Verlag, 1983, ISBN 3-13-217404-1 , II. Dicarbonic acid derivatives with orthocarbonic acid functions: a) with an orthocarbonic acid function, p. 1204 , doi : 10.1055 / b-0035-112295 (can be seen in the preview).
- ↑ a b Alan R. Katritzky, Richard JK Taylor (Ed.): Comprehensive Organic Functional Group Transformations II . 2nd Edition. Elsevier Science, 2004, ISBN 978-0-08-044256-3 , pp. 955 ( limited preview in Google Book Search).