1-hydroxy-7-azabenzotriazole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
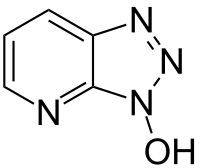
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-hydroxy-7-azabenzotriazole | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 4 N 4 O | |||||||||||||||
| Brief description |
light yellow solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 136.11 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
213–216 ° C (decomposition) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1-Hydroxy-7-azabenzotriazole (HOAt) is a triazole that is used in organic chemistry and biochemistry as an additive for peptide synthesis and generally for the coupling of amino acids . HOAt reduces the racemization . Alternatively, hydroxybenzotriazole (HOBT), HATU , HBTU , TBTU , N -hydroxysuccinimide or ethyl 2-cyano-2- (hydroxyimino) acetate (Oxyma) are used. HOAt is also used for cross-linking of proteins used.
Individual evidence
- ↑ a b c d e Data sheet 1-Hydroxy-7-azabenzotriazole, 98% from Sigma-Aldrich , accessed on August 1, 2014 ( PDF ).
- ↑ Holger Wenschuh, Michael Beyermann, Hanka Haber, Joachim K. Seydel, Eberhard Krause, Michael Bienert, Louis A. Carpino, Ayman El-Faham, Fernando Albericio: Stepwise Automated Solid Phase Synthesis of Naturally Occurring Peptaibols Using FMOC Amino Acid Fluorides. In: The Journal of Organic Chemistry. 60, 1995, pp. 405-410, doi : 10.1021 / jo00107a020 .
- ↑ GB Fields: Introduction to peptide synthesis. In: Current protocols in molecular biology / edited by Frederick M. Ausubel ... [et al.]. Chapter 11 August 2002, S. Unit 11.15, doi : 10.1002 / 0471142727.mb1115s59 . PMID 18265296 .
- ↑ Louis A Carpino, Ayman El-Faham: The diisopropylcarbodiimide / 1-hydroxy-7-azabenzotriazole system: Segment coupling and stepwise peptide assembly. In: Tetrahedron. 55, 1999, pp. 6813-6830, doi : 10.1016 / S0040-4020 (99) 00344-0 .
- ↑ J. Klose, A. El-Faham, P. Henklein, LA Carpino, M. Bienert: Addition of HOAt dramatically improves the effectiveness of pentafluorophenyl-based coupling reagents. In: Tetrahedron Letters . 40, 1999, p. 2045, doi : 10.1016 / S0040-4039 (99) 00089-1 .
- ↑ R. Subirós-Funosas, R. Prohens, R. Barba, A. El-Faham, F. Albericio: Oxyma: an efficient additive for peptide synthesis to replace the benzotriazoles-based HOBt and HOAt with a lower risk of explosion. In: Chemistry. Volume 15, number 37, September 2009, pp. 9394-9403, doi : 10.1002 / chem.200900614 . PMID 19575348 .
- ↑ C. Bich, S. Maedler, K. Chiesa, F. DeGiacomo, N. Bogliotti, R. Zenobi : Reactivity and applications of new amine reactive cross-linkers for mass spectrometric detection of protein-protein complexes. In: Analytical chemistry. Volume 82, Number 1, January 2010, pp. 172-179, doi : 10.1021 / ac901651r . PMID 19994840 .


