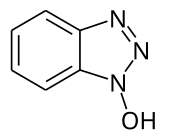
1-hydroxybenzotriazole
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | 1-hydroxybenzotriazole | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 6 H 5 N 3 O | |||||||||
| Brief description |
Solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass |
|
|||||||||
| Physical state |
firmly |
|||||||||
| density |
1.438 g cm −3 |
|||||||||
| Melting point |
|
|||||||||
| Vapor pressure |
0.009 Pa |
|||||||||
| pK s value |
4.6 |
|||||||||
| solubility |
4.201 g l −1 in water (29 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
1-Hydroxybenzotriazole (also often abbreviated as HOBt ) is a derivative of benzotriazole which is substituted in the 1-position by a hydroxyl group . As a result of the structure with a triazole ring and a nitrogen-oxygen bond, the connection is thermally unstable and is considered to be explosive. The most important application is as a reagent in peptide synthesis . The compound contains the basic structure for other benzotriazole derivatives important in peptide synthesis, such as TBTU and HBTU .
Presentation and extraction
1-Hydroxybenzotriazole 2 is produced by the oxidation of benzotriazole 1 with hydrogen peroxide in the presence of sodium tungstate .
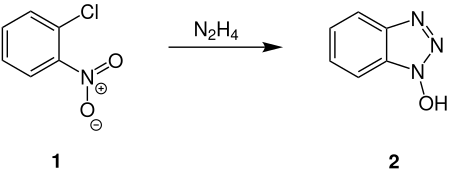
Another synthesis variant is the cyclization of 2-chloronitrobenzene 1 using hydrazine hydrate .
properties
1-Hydroxybenzotriazole can exist in two tautomeric forms. The equilibrium lies between the N -oxide and N -hydroxy tautomers. A third possible tautomer as 1-oxy-2 H -benzotriazole is thermodynamically unstable and plays practically no role. The equilibrium position depends on the type of solvent. The N -oxide tautomer is preferred in an aqueous medium ; the N -hydroxy tautomer dominates in organic solvents such as methanol , ethanol , acetone , formamide and dimethyl sulfoxide . The N -oxide tautomer crystallizes from aqueous methanol and is obtained as a monohydrate with 11.7% water . The colorless crystals have a melting point of 159–161 ° C and form a monoclinic crystal lattice with the space group P2 1 / C.) The N -hydroxy tautomer can be obtained from anhydrous ethanol / ether mixtures. It also forms colorless crystals that melt at 169–170 ° C. The crystal lattice is monoclinic with the space group C2 / c.
Above the melting point, a strongly exothermic decomposition with a heat of decomposition of −2259 kJ kg −1 or −305 kJ mol −1 is observed. In Germany, 1-hydroxybenzotriazole is classified as an explosive substance in accordance with the regulations of the Explosives Act. The anhydrous substance is assigned to substance group B. Mixtures with a water content between 20% and 47% still fall into substance group C. In the steel sleeve test , the limit diameter of the perforated plate for anhydrous material is 10 mm. The limit diameter decreases with increasing water content. With a water content of 50%, however, a relevant limit diameter of 2 mm in terms of the Explosives Act is observed. The connection is sensitive to impact . An impact energy of 10 J. is sufficient for anhydrous 1-hydroxybenzotriazole. With a water content of up to 20%, an impact energy of 20 J is necessary. HOBt is only available commercially as a hydrate, as it is classified as a class 4.1 hazardous material as a combustible solid. Pure HOBt falls under dangerous goods class 1.3C explosives. The resulting increased effort for transport and storage is mostly uneconomical.
use
1-Hydroxybenzotriazole (abbreviated to HOBt) is an important reagent in peptide synthesis, both in solution and in the solid phase. HOBt is used to produce a so-called active ester from a carboxylic acid (usually a protected amino acid ) activated by a carbodiimide . These active esters react with amines to form the corresponding peptide bonds (or better amides ). The reaction with HOBt as an auxiliary reagent is characterized by a low-racemization reaction, takes place at room temperature and generally has better yields compared to direct couplings. Alternatively, HATU , HBTU , TBTU or 1-hydroxy-7-azabenzotriazole (HOAT) are used.
literature
- WC Chan, PD White: Fmoc Solid Phase Peptide Synthesis . Reprint 2004, Oxford University Press, ISBN 0-19-963724-5 .
Individual evidence
- ↑ a b c Entry on 1-hydroxybenzotriazole in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c ECHA Substance Information , accessed on November 27, 2018.
- ↑ a b c d Bosch, R .; Jung, G .; Winter, W .: Benzotriazole 1-oxide and 1-hydroxybenzotriazole, C 6 H 5 N 3 O: structures of both tautomeric forms in Acta Crystallogr. Sect. C 39 (1983) 1089-1092, doi : 10.1107 / S0108270183007490 .
- ↑ a b c d e Pfister-Guillouzo, G .; Gracian, F .; Paez, YES; Gomez, CG; Elguero, J .: Study of 1-hydroxybenzotriazole / benzotriazole N-oxide tautomerism in gas phase by photoelectron spectroscopy in Spectrochim. Acta A51 (1995) 1801-1807, doi : 10.1016 / 0584-8539 (95) 01441-V .
- ↑ Boyle, FT; Jones, RAY: Azoles N-oxides. Part I. The tautomerism of benzotriazole 1-oxide and its 4- and 6-nitro-derivatives with the corresponding 1-hydroxybenzotriazoles in J. Chem. Soc. Perkin Trans. 2, 1973, 160-164, doi : 10.1039 / P29730000160 .
- ^ Koppel, I .; Koppel, J .; Leito, I .; Pihl, V .; Grehn, L .; Ragnarsson, U .: in J. Chem. Res. 11 (1993) 3008-3028.
- ↑ Entry on 1-hydroxybenzotriazole in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ 1-Hydroxybenzotriazole data sheet from Sigma-Aldrich , accessed on January 25, 2020 ( PDF ).
- ↑ Kruglik, AP; Rakhman'ko, EM; Asratyan, GV: in J. Appl. Chem. USSR (Engl. Transl.) 62 (1989) 2385-2386, 2216-2218.
- ↑ Fu, Jie; Yang, ying; Zhang, Xue-Wei; Mao, Wen-Jun; Zhang, Zhi-Ming; Zhu, Hai-Liang: Discovery of 1H-benzo [d] [1,2,3] triazol-1-yl 3,4,5-trimethoxybenzoate as a potential antiproliferative agent by inhibiting histone deacetylase . In: Bioorganic & Medicinal Chemistry 18 (2010) 8457-8462, doi : 10.1016 / j.bmc.2010.10.049 .
- ↑ a b Fruchier, A .; Elguero; J .; Hagarty, AF; McGarthy, DG: NMR studies in the heterocyclic series. XX — a carbon-13 NMR study of the structures of N -hydroxybenzotriazole and its acylated derivatives in Org. Magn. Reson. 13 (1980) 339-342, doi : 10.1002 / mrc.1270130508 .
- ↑ a b Schilf, W .; Stefaniak, L .; Witanowski, M .; Webb, GA: 15 N NMR studies of the tautomeric equilibrium of some 1-hydroxybenzotriazoles in Magn. Reson. Chem. 23 (1985) 181-184, doi : 10.1002 / mrc.1260230310 .
- ↑ a b Wehrstedt, KD; Wandrey, PA; Heitkamp, D .: Explosive properties of 1-hydroxybenzotriazoles in J. Hazard. Mat. A126 (2005) 1-7, doi : 10.1016 / j.jhazmat.2005.05.044 .
- ↑ a b BAM notification No. 416 of May 21, 2002, No. 435 of January 23, 2003 and No. 471 of June 6, 2010 (PDF; 345 kB).
- ↑ a b c UNECE ECOSOC Sub-Committee of Experts on the Transport of Dangerous Goods (TDG) - UN / SCETDG / 29 / INF.22 - (Germany) - Classification of 1-hydroxybenzotriazole, anhydrous (HOBt), under a division of Class 1 (PDF; 136 kB).
- ↑ Wolfgang König, Rolf Geiger: A new method for the synthesis of peptides: Activation of the carboxyl group with dicyclohexylcarbodiimide with the addition of 1-hydroxy-benzotriazoles. In: Chemical Reports . 103, 1970, p. 788, doi : 10.1002 / cber.19701030319 .
- ↑ R. Subirós-Funosas, R. Prohens, R. Barba, A. El-Faham, F. Albericio: Oxyma: an efficient additive for peptide synthesis to replace the benzotriazoles-based HOBt and HOAt with a lower risk of explosion. In: Chemistry. Volume 15, number 37, September 2009, pp. 9394-9403, doi : 10.1002 / chem.200900614 . PMID 19575348 .