Benzotriazole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Benzotriazole | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 5 N 3 | |||||||||||||||
| Brief description |
beige solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 119.13 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.36 g cm −3 |
|||||||||||||||
| Melting point |
100 ° C |
|||||||||||||||
| boiling point |
350 ° C |
|||||||||||||||
| Vapor pressure |
5.3 Pa (20 ° C) |
|||||||||||||||
| pK s value |
8.37 |
|||||||||||||||
| solubility |
20 g l −1 in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
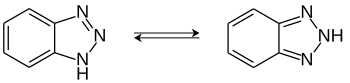
1 H -Benzotriazole is an organic chemical with a 1,2,3-triazole structure that is used as a complexing agent . A structurally isomeric compound with which 1 H -benzotriazole is in tautomeric equilibrium is 2 H -benzotriazole.
properties
1 H -benzotriazole is a white, crystalline powder which, after purification by repeated sublimation, melts at 100.5 ° C. in a high vacuum. The compound crystallizes in a monoclinic crystal lattice. The vapor pressure relevant for sublimation is in the temperature range between 53.4 ° C and 71.7 ° C between 90 mPa and 640 mPa and can be calculated using the equation lg (p / Pa) = 5159.5 (T / K) −1 + 14.77. The enthalpy of sublimation is at 25 ° C. Δ sub H = 98.15 kJ / mol, at 63.4 ° C. Δ sub H = 96.30 kJ / mol.
The compound decomposes strongly exothermically above the melting point. Distillation of the compound is therefore not advisable. A source from 1956 reports the explosive decomposition of a one tonne batch during distillation at 160 ° C and 2.5 hPa. A thermoanalytical investigation showed a decomposition reaction with a very high reaction heat of −1590 kJ from 240 ° C / kg. Despite the high heat of decomposition, the compound is not explosive within the meaning of the Explosives Act , as none of the tests relevant for classification, such as B. the BAM 50/60 steel pipe test or the Koenen test (with 1 mm bore of the nozzle plate: type O) delivers a positive result.
A tautomeric equilibrium can be formulated between 1 H -benzotriazole and 2 H -benzotriazole . However, experimental measurements and quantum chemical calculations show that in the solid phase and in solution this equilibrium is practically on the side of the 1 H -benzotriazole. This is the energetically more favorable tautomer. In the photochemically excited triplet state , however, the 2 H tautomer is energetically favored or more stable.
Benzotriazole has amphiprotic properties, so it can be protonated in an acidic medium or deprotonated in a basic medium. The protolysis constants are pK a1 = 0.42 and pK a2 = 8.27.
Extraction and presentation
It is produced by the diazotization of o-phenylenediamine with nitrite and (acetic) acid.
use
Benzotriazole is used as a corrosion protection agent, especially for copper in coolants , antifreeze , de-icing agents and in descaling tablets. It serves as a silver protection in dishwashing detergents . In the industry it is in cooling lubricants used metalworking. In photographic developers it is used to reduce fogging on the film.
Benzotriazole derivatives (e.g. 5-methyl-1H-benzotriazole ) are used, for example, as antifreeze agents , UV filters , in solar cells or in medical applications.
Environmental relevance
Benzotriazole is relatively soluble in water and difficult to break down. It is therefore only eliminated to a small extent in sewage treatment plants and ends up in large quantities in rivers and lakes. In European rivers, concentrations in the three to four-digit nanogram per liter range are typically measured.
safety instructions
Benzotriazole was included in the EU's ongoing action plan ( CoRAP ) in 2014 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of benzotriazole were concerns about environmental exposure and as a potential endocrine disruptor . The re-evaluation has been running since 2016 and is carried out by Germany . In order to be able to reach a final assessment, further information was requested.
Web links
- Adhesion promoter based on 1 H -benzotriazole for aluminum and copper bonds (PDF; 3.91 MB)
- Corrosion inhibitors
Individual evidence
- ↑ Entry on BENZOTRIAZOLE in the CosIng database of the EU Commission, accessed on March 21, 2020.
- ↑ a b c d e Benzotriazole data sheet from Sigma-Aldrich , accessed on June 11, 2011 ( PDF ).
- ↑ a b c d Entry on 1H-Benzotriazole in the SRC PhysProp Database , accessed on September 11, 2012.
- ↑ a b Entry on benzotriazole in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ KWF Kohlrausch, R. Seka; Raman Effect and Constitutional Problems, XIII. Mitteil .: Naphthalene-like condensed hetero-bicyclics ; Chemical Reports 71 (1938), 1563-1570 doi : 10.1002 / cber.19380710803
- ↑ A. Escande, JL Galigné, J. Lapasset: Structure cristalline et moléculaire du benzotriazole ; Acta Cryst. 1974 B 30: 1490-1495; doi : 10.1107 / S0567740874005139
- ↑ P. Jimenez, MV Roux, C. Turrion: Thermochemical properties of N-heterocyclic compounds II. Enthalpies of combustion, vapor pressures, enthalpies of sublimation, and enthalpies of formation of 1,2,4-triazole and benzotriazole ; J. Chem. Thermodyn. 1989, 21: 759-764; doi : 10.1016 / 0021-9614 (89) 90060-8
- ↑ R. Sabbah, L. Perez; Energetics of Intramolecular Bonds in 1H-1,2,4-Triazole and 1H-Benzotriazole , in: Austr. J. Chem. 1999 , 52 , 235-240; doi : 10.1071 / C99006 .
- ↑ a b c M. Malow, KD Wehrstedt, S. Neuenfeld: On the explosive properties of 1H-benzotriazole and 1H-1,2,3-triazole . Tetrahedron Letters (2007) 48: 1233-1235; doi : 10.1016 / j.tetlet.2006.12.046
- ↑ Anon., Chem. Eng. News, 1956, 34, 2450
- ↑ F. Tomas; JLM Abboud; J. Laynez; R. Notario; L. Santos; SO Nilsson; RM Claramunt; J Elguero: Tautomerism and aromaticity in 1,2,3-triazoles: The case of Benzotriazole in J. Am. Chem. Soc. 111 (1989) 7348-7353. doi : 10.1021 / ja00201a011
- ↑ a b J. Catalan; P. Perez: Theoretical and experimental evidence on the structure of benzotriazole in its first triplet electronic state in Chem. Phys. Letters 404 (2005) 304-308. doi : 10.1016 / J.cplett.2005.01.107
- ↑ AR Katritzky; K. Yannakopoulou; E. Anders; J. Stephens; M. Szafran: Ab initio and semiempirical calculations on the tautomeric equilibria of N-unsubstituted and N-substituted benzotriazoles in J. Org. Chem. 55 (1990 5683-5687). doi : 10.1021 / jo00309a009
- ↑ H. Wang, C. Burda, G. Persy, J. Wirz: Photochemistry of 1H-Benzotriazole in Aqueous Solution: A Photolatent Base in J. Am. Chem. Soc. 122 (2000) 5849-5855; doi : 10.1021 / ja994464c
- ↑ US Patent 4,299,965
- ↑ a b entry on 1H-benzotriazole. In: Römpp Online . Georg Thieme Verlag, accessed on May 1, 2019.
- ↑ Data sheet 5-Methyl-1H-benzotriazole from Sigma-Aldrich , accessed on February 2, 2019 ( PDF ).
- ↑ K. Bajaj, R. Sakhuja: Benzotriazoles: Much More Than Just Synthetic Heterocyclic Chemistry . In: JC Monbaliu (Ed.): The Chemistry of Benzotriazole Derivatives. Topics in Heterocyclic Chemistry , edition 43.Springer, Cham 2015, doi: 10.1007 / 7081 2015 198 , ISBN 978-3-319-31552-2 , ISBN 978-3-319-31554-6 .
- ^ W. Giger , C. Schaffner, HP. Kohler (2006): Benzotriazole and Tolyltriazole as Aquatic Contaminants. 1. Input and Occurrence in Rivers and Lakes . Environ. Sci. Technol. 40, pp. 7186-7192. PMID 17180965 .
- ↑ Antifreeze discovered in the North Sea. In: welt.de . November 30, 2011, accessed May 1, 2015 .
- ↑ Robert Loos, Bernd Manfred Gawlik, Giovanni Locoro, Erika Rimaviciute, Serafino Contini, Giovanni Bidoglio: EU-wide survey of polar organic persistent pollutants in European river waters . In: Environmental Pollution . 2009. doi: 10.1016 / j.envpol.2008.09.020 .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Benzotriazole , accessed on March 26, 2019.