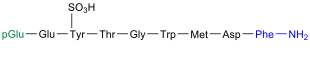Nonapeptides
Nonapeptides are peptides that consist of nine amino acid building blocks and thus belong to the oligopeptides . The term "amino acid building block" comes from the fact that the individual amino acids are linked to one another via peptide bonds . Such a bond is formed by splitting off water between the amino group of one amino acid and the carboxy group of another amino acid. Like almost all peptides, the nonapeptides are often pharmacologically active.
Linear nonapeptides
In linear nonapeptides, the individual building blocks of the amino acids are linked to one another in a chain via eight peptide bonds.
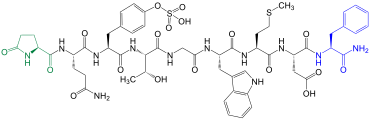
Bradykinin
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
 Bradykinin comes in blood plasma before |
The kinin bradykinin is one of the tissue hormones and was discovered in 1948 by a group led by the Brazilian Maurício Rocha e Silva (1910–1983) in the blood plasma when the venom of the lance viper Bothrops jararaca was injected into a dog. As is usual with these kinins, it has an antihypertensive effect, increases the vascular permeability and leads to the contraction of the smooth muscles of the intestines , uterus and bronchi . This nonapeptide is released when the body responds to trauma or injury. In the process, messenger substances are released by the leukocytes , which indicates that bradykinin is involved, among other things, in the sensation of pain and inflammation. Thus, as a result of an injection of bradykinin z. B. Pain felt and swelling visible.
DSIP
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
 |
DSIP is the abbreviation for D elta S leep I nducing P eptide, which means something like sleep-inducing peptide. In 1963 it was discovered by the Swiss physiologist Marcel Monnier (1907–1996) that it is possible to transfer sleep from one rabbit to another rabbit. To do this, sleep was induced in one rabbit by electrical stimulation of the thalamus and transferred to another by exchanging body fluids (i.e. humoral). The search for the substance that is responsible for this transmission then began. 1976 could this substance, the nonapeptide DSIP, permanently from cerebral , venous be isolated blood of rabbits. DSIP and DSIP-like peptides come in different mammals and cold-blooded vertebrates before. In the respective organism, the peptides are involved in various regulatory mechanisms, such as:
- Reduction of the basal corticotropin level
- Secretion of luteinizing hormone
- Lowering the stress level
- In the immune response as an immunomodulatory factor or anti-tumor agent
- Normalization of blood pressure
- Suppression of alcohol and opiate addiction
Although the peptide is called the “ sleep inducing peptide, ” its effects on sleep are controversial.
Phyllocaerulein
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
 Phyllocaerulein the frog comes in the skin Phyllomedusa sauvagei ago |
The neuropeptide phyllocaerulein (also: phyllocerulein) was isolated from the skin of the frog Phyllomedusa sauvagei in 1969 by a group led by the Italian pharmacologist Vittorio Erspamer (1909–1999) . Phyllocaerulein is very similar to the decapeptide caerulein and has the same effect. This means that the nonapeptide influences the blood pressure, contraction of the gallbladder leads and the gastric acid and pancreatic - secretion increases.
Teprotid
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
 Teprotide comes in the venom of the fer de lance Bothrops jararaca ago |
In 1970, a group led by the Brazilian pharmacologist Sérgio Henrique Ferreira (1934-2016) was able to isolate peptides from the venom of the lance viper Bothrops jararaca that potentiate bradykinin because they are ACE inhibitors . One of these peptides was later called Teprotid. Due to little commercial interest, this material has not been further developed. Teprotid was too expensive and could not be administered orally .
Cyclic nonapeptides
Cyclic nonapeptides are circular peptides . The formation of a ninth peptide bond can create a monocyclic compound . A cyclization is also carried further covalent bonds, such as a disulfide bond in oxytocin and arginine vasopressin possible.
Arginine vasopressin
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
 Arginine vasopressin is in the pituitary gland before |
In 1927 Oliver Kamm and colleagues succeeded in isolating two extracts from the pituitary gland . Activities typical of oxytocin were demonstrated by one extract and e.g. B. increased blood pressure. This component was called vasopressin.
A few years later it was found that there are two types of vasopressin: arginine vasopressin, which is found in humans, cattle, sheep, horses and cats, and lysine vasopressin, which is found in pigs. The difference between these two variants is that lysine vasopressin contains a lysine component instead of an arginine component .
In 1958 a group led by the American biochemist Vincent du Vigneaud (1901–1978) succeeded in synthesizing the nonapeptide arginine vasopressin . In addition to its ability to increase blood pressure, arginine vasopressin generally helps to maintain homeostasis ( water retention , increase in arousal ...) and is therefore also referred to as a self-serving peptide .
Oxytocin
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
 Oxytocin is used in the pituitary gland before |
The British biochemist Henry Dale (1875–1968) discovered in 1906 that there are substances in the pituitary gland which, among other things, cause the uterus to contract . The neuropeptide oxytocin, which belongs to the proteohormones, was discovered. In 1953, the amino acid sequence of the nonapeptide was worked out by the American biochemist Vincent du Vigneaud (1901–1978). In the same year he also synthesized the substance. In addition to the contraction of the uterus, the mode of action of oxytocin z. B. the positive influence on the social interaction between mother and child is characteristic. Due to its generally positive effect on social behavior, oxytocin is also known as a disinterested peptide . This peptide also lowers blood pressure and cortisol levels. It can have a sedating effect and lead to weight gain and improved wound healing.
See also
Individual evidence
- ↑ a b c d e f G. D. Fassman (Ed.): Handbook of Biochemistry an Molecular Biology. (= Proteins. Volume I). 3. Edition. CRC Press, Cleveland 1976, ISBN 0-87819-504-1 , pp. 1-108.
- ^ A b M. Rocha e Silva, WT Beraldo, G. Rosenfeld: Bradykinin, a Hypotensive and Smooth Muscle Stimulating Factor Released from Plasma Globulin by Snake Venoms and by Trypsin. In: American Journal of Physiology . Volume 156, No. 2, 1949, pp. 261-273. PMID 18127230 .
- ↑ BJ Hawgood: Maurício Rocha E Silva MD: Snake venom, bradykinin and the rise of autopharmacology. In: Toxikon. Volume 35, No. 11, 1997, pp. 1569-1580, doi: 10.1016 / S0041-0101 (97) 00008-1 .
- ↑ a b H.-D. Jakubke: peptides. Spektrum Akademischer Verlag , Heidelberg / Berlin / Oxford 1996, ISBN 3-8274-0000-7 , p. 65.
- ↑ JE Taylor, FV DeFeudis, JP Moreau: bradykinin antagonists: Therapeutic perspectives. In: Drug Development Research . Volume 16, No. 1, pp. 1-11, doi: 10.1002 / gdr.430160102 .
- ^ CW Parker: Mediators: Release and Function. In: WE Paul: Fundamental Immunology. Raven Press , New York 1984, ISBN 0-89004-923-8 .
- ↑ a b G. A. Schoenenberger, PF Maier, HJ Tobler, M. Monnier: A naturally occurring delta-EEG enhancing nonapeptide in rabbits - X. Final isolation, characterization and activity test. In: Pflüger's archive . Volume 369, No. 2, pp. 99-109, doi: 10.1007 / BF00591565 .
- ^ University of Basel: Prof. Marcel Monnier. In: University of Basel 1460–2010 - Review of the last 50 years of the Institute for Physiology. 2010, accessed June 29, 2017.
- ↑ M. Monnier, T. Koller, S. Graber: Humoral Influences of Induced Sleep and Arousal upon Electrical Brain Activity of Animals with Crossed Circulation. In: Experimental Neurology . Volume 8, No. 3, 1963, pp. 264-277, doi: 10.1016 / 0014-4886 (63) 90036-0 .
- ↑ a b V. M. Kovalzon, TV Strekalova: Delta sleep-inducing peptide (DSIP): a still unresolved riddle. In: Journal of Neurochemistry . Volume 97, No. 2, 2006, pp. 303-309, doi: 10.1111 / j.1471-4159.2006.03693.x .
- ^ AA Borbély, I. Tobler: Endogenous Sleep-Promoting Substances and Sleep Regulation. In: Physiological reviews . Volume 69, No. 2, 1989, pp. 605-670. PMID 2564687 .
- ↑ a b c d A. Anastasi, G. Bertaccini, JM Cei, G. Caro, V. Erspamer, M. Impicciatore: Structure and pharmacological actions of phyllocaerulein, a caerulein-like nonapeptide: its occurrence in extracts of the skin of Phyllomedusa sauvagei and related Phyllomedusa species. In: British Journal of Pharmacology . Volume 37, No. 1, 1969, pp. 198-206, doi: 10.1111 / j.1476-5381.1969.tb09538.x .
- ↑ G. Bertaccini, G. de Caro, R. Endean, V. Erspamer, M. Impicciatore: The action of caerulein on the systematic arterial blood pressure of some experimental animals. In: British Journal of Pharmacology . Volume 33, No. 1, 1968, pp. 59-71, doi: 10.1111 / j.1476-5381.1968.tb00474.x .
- ↑ G. Bertaccini, G. de Caro, R. Endean, V. Erspamer, M. Impicciatore: The actions of caerulein on the smooth muscle of the gastrointestinal tract and the gall bladder. In: British Journal of Pharmacology . Volume 34, No. 2, 1968, pp. 291-310, doi: 10.1111 / j.1476-5381.1968.tb07052.x .
- ^ V. Erspamer, GF Erspamer, C. Severini, RL Potenza, D. Barra, G. Mignogna, A. Bianchi: Pharmacological studies of 'sapo' from the frog Phyllomedusa bicolor skin: A drug used by the Peruvian Matses Indians in shamanic hunting practices. In: Toxicon . Volume 31, No. 9, 1993, pp. 1099-1111, doi: 10.1016 / 0041-0101 (93) 90125-3 .
- ↑ a b c S. H. Ferreira, DC Bartelt, LJ Greene: Isolation of Bradykinin-Potentiating Peptides from Bothrops jararaca Venom. In: Biochemistry . Volume 9, No. 13, 1970, pp. 2583-2593, doi: 10.1021 / bi00815a005 .
- ↑ MA Ondetti, NJ Williams, EF Sabo, J. Pluscec, ER Weaver, O. Kocy: Angiotensin-Converting Enzyme Inhibitors from the Venom of Bothrops jararaca. Isolation, Elucidation of Structure, and Synthesis. In: Biochemistry . Volume 10, No. 22, 1971, pp. 4033-4039, doi: 10.1021 / bi00798a004 .
- ^ DW Cushman, MA Ondetti: History of the Design of Captopril and Related Inhibitors of Angiotensin Converting Enzyme. In: Hypertension . Volume 17, No. 4, 1991, pp. 589-592, doi: 10.1161 / 01.HYP.17.4.589 .
- ↑ K. Nemec, M. Schubert-Zsilavecz: From Teprotid to Captopril. Rational design of ACE inhibitors. In: Pharmacy in our time . Volume 32, No. 1, 2003, pp. 11-16, doi: 10.1002 / pauz.200390001 .
- ↑ a b O. Kamm, TB Aldrich, IW Grote, LW Rowe, EP Bugbee: THe active principles of the posterior lobe of the pituitary glad. I. The demonstration of the presence of two active principles. II. The separation of the two principles and their concentration in the form of potent solid preperations. In: Journal of the American Chemical Society . Volume 50, No. 2, 1928, pp. 573-601, doi: 10.1021 / ja01389a050 .
- ↑ a b A. W. Bourne, JH Burn: The action of oxytocin and vasopressin on the uterus in labor. In: The Lancet . Volume 212, No. 5484, 1928, pp. 694-695, doi: 10.1016 / S0140-6736 (00) 84559- .
- ^ A b E. A. Popenoe, HC Lawler, V. du Vigneaud: Partial purification and amino acid content of vasopressin from hog piturity glands. In: Journal of the American Chemical Society . Volume 74, No. 14, 1952, p. 3713, doi: 10.1021 / ja01134a528 .
- ↑ W. Sneader: Drug Discovery: A History. John Wiley & Sons , Chichester, 2005, ISBN 0-470-01553-5 , p. 168, doi: 10.1002 / 0470015535.ch14 .
- ^ V. du Vigneaud, DT Gish, PG Katsoyannis, GP Hess: Synthesis of the Pressor-Antidiuretic Hormone, Arginine-Vasopressin. In: Journal of the American Chemical Society . Volume 80, No. 13, 1958, pp. 33553-358, doi: 10.1021 / ja01546a040 .
- ↑ a b J.-J. Legros: Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying – yang neurohormones? In: Psychoneuroendocrinology . Volume 26, No. 7, 2001, pp. 649-655, doi: 10.1016 / S0306-4530 (01) 00018-X .
- ↑ a b H. H. Dale: On some physiological actions of ergot. In: The Journal of Physiology . Volume 34, No. 3, 1906, pp. 163-206, doi: 10.1113 / jphysiol.1906.sp001148 .
- ↑ V. du Vigneaud, C. Ressler, S. Trippett: The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. In: The Journal of biological chemistry . Volume 205, No. 2, 1953, pp. 949-957. PMID 13129273 .
- ↑ V. du Vigneaud, C. Ressler, CJM Swan, CW Roberts, PG Katsoyannis, S. Gordon: The Synthesis of an Octapeptide Amide with the Hormonal Activity of Oxytocin. In: Journal of the American Chemical Society . Volume 75, No. 19, pp. 4879-4880, doi: 10.1021 / ja01115a553 .
- ↑ a b K. Uvnäs-Moberg: Oxytocin may mediate the benefits of positive social interaction and emotions. In: Psychoneuroendocrinology . Volume 23, No. 8, 1998, pp. 819-835, doi: 10.1016 / S0306-4530 (98) 00056-0 .