Cholestyramine
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|||||||||
| General | |||||||||
| Surname | Cholestyramine | ||||||||
| other names |
|
||||||||
| CAS number | 11041-12-6 | ||||||||
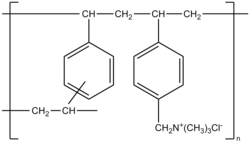
| Monomers / partial structures | Vinylbenzene and 2% divinylbenzene with trimethylbenzyl quaternary ammonium groups | ||||||||
| ATC code | |||||||||
| DrugBank | DB01432 | ||||||||
| Brief description |
White to almost white, fine, hygroscopic powder |
||||||||
| Drug information | |||||||||
| Drug class |
Lipid lowering agents , bile acids - complexing agents |
||||||||
| Mechanism of action |
Absorption of bile acids |
||||||||
| properties | |||||||||
| solubility |
Insoluble in water , dichloromethane and ethanol |
||||||||
| safety instructions | |||||||||
|
|||||||||
| Toxicological data | |||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Colestyramine is an inhibitor of the absorption of cholesterol . It is used for hypercholesterolemia , either as the only active ingredient or with a statin . It is used as a diagnostic agent for chologene diarrhea or is used in the treatment of the same if it is an operative consequence.
Chemical properties
Colestyramine is a strongly basic anion exchanger . It is made up of vinylbenzene monomers ( styrene ) and a small proportion (two percent) of divinylbenzene monomers. The polymer chains are connected to one another via the divinylbenzene units , resulting in a two-dimensional network. Quaternary trimethylbenzylammonium groups , which are present in the drug form as chloride salt, are inserted into this polymer structure . The average molar mass of a polymer is 1 · 10 6 g · mol −1 .
pharmacology
Pharmacokinetics
Colestyramine is not enzymatically decomposable. It is highly hydrophilic , but insoluble in water and a very large macromolecule . Therefore it cannot be absorbed by the intestine and is not subject to metabolism .
Pharmacodynamics
Colestyramine only works in the intestine. The active substance is to chloride - anions bound to the bile acids to be replaced. Colestyramine has a high affinity for bile acids in the gastrointestinal tract . The combination of cholestyramine and the bile acids can hardly be dissolved in the intestine. Since the colestyramine polymers bind a large number of bile acid molecules and the macromolecule becomes even larger, the cholestyramine bile acid molecule cannot be reabsorbed and is excreted through the faeces .
By taking cholestyramine, bile acid is continuously withdrawn from the body, as the bile acids excreted through the bile duct into the intestine are usually later reabsorbed in the back of the small intestine for the most part. The bile acids are transported back to the liver via this enterohepatic cycle . When the liver cannot get bile acids back from the intestines, it begins to produce new bile acids by upregulating the enzyme cholesterol 7α-hydroxylase (CYP7A1). As a result, the liver produces more bile acids and thus uses more cholesterol , since cholesterol is the only precursor of bile acids.
Dosage form
Medicines containing cholestyramine are available in the form of granules , powders and chewable tablets. They are taken orally with plenty of water.
application areas
Colestyramine is used as an adjuvant therapy to lower high levels of LDL - cholesterol in the blood. It is used both as a monotherapy during the diet and in combination with an HMG-CoA reductase inhibitor ( statin ). Colestyramine is also used therapeutically for chologetic diarrhea (diarrhea due to too much bile acids in the intestine) and also for itching and jaundice due to partial bile duct obstruction. Another area of application is the interruption of the enterohepatic circulation in the event of drug intoxication. By administering cholestyramine, drugs that are subject to the enterohepatic circulation such as B. Digitoxin can be eliminated more effectively.
Contraindications
Colestyramine must not be taken if there is a hypersensitivity to the active ingredient, in the case of intestinal obstruction or obstruction of the bile duct . Since colestyramine can reduce the absorption of fat-soluble vitamins ( A , D , E , K ), the use during pregnancy is carefully weighed and monitored by the attending physician if there is no safe alternative to lowering the cholesterol level and the cholesterol level urgently needs lowering .
Interactions
Interactions due to intake too close in time
The anion-exchanging properties of cholestyramine ensure an extraordinarily large variety of interactions , both reductions in absorption and delays in absorption are not uncommon when drugs are administered orally at the same time. In general, it is strongly advised to take other medicines at least one hour before taking cholestyramine or at least four hours after taking cholestyramine. Particularly at risk for an interaction with colestyramine are u. a. Phenylbutazone ( anti-inflammatory drug ), hydrochlorothiazide ( diuretic drug ), the antibiotics tetracycline and penicillin G , as well as phenobarbital (for epilepsy ) and thyroid drugs .
Interactions through participation in the enterohepatic cycle
Drugs that are subject to the enterohepatic cycle can be influenced in their pharmacokinetics by cholestyramine even if they are taken at different times , provided that they have a significant affinity for cholestyramine. The blood levels of these drugs are then reduced. Conversely, the sudden discontinuation of cholestyramine can cause the plasma concentration of drugs to rise to a toxic level, if the dose was adjusted to the losses caused by cholestyramine. For this reason, the colestyramine dose should never be changed without consulting your doctor.
Interactions with anticoagulant drugs
Patients taking cholestyramine and being treated with anti- coagulants (e.g. warfarin or phenprocoumon ) need to monitor their blood clotting levels even more closely . Bile acid binding drugs reduce the absorption of vitamin K , which increases the risk of bleeding because fewer coagulation factors are produced when less vitamin K is ingested from the diet. The anticoagulant effect of anticoagulants is also impaired by colestyramine.
Interactions rated as particularly significant
- Particular attention should be paid to the possible interaction of cholestyramine with oral anticoagulants (see above), since too strong an anticoagulation leads to a tendency to bleed , while too weak an anticoagulation can lead to thrombosis .
- Digitoxin , a cardiac glycoside for the treatment of heart failure , is reduced in its plasma level as soon as it meets colestyramine through its participation in the enterohepatic circulation , which can result in an insufficient effect. If the patient is adjusted to an increased dose (to compensate for the losses caused by cholestyramine), sudden discontinuation of cholestyramine can lead to life-threatening digitoxin poisoning.
- Colestyramine can lower the plasma level of estrogens because it removes them from the enterohepatic circulation. If estrogens are used for contraception in women, the contraceptive (contraceptive) effect of the birth control pill may be reduced , with the possible consequence of an unplanned pregnancy.
- Colestyramine can cause a deficiency in fat-soluble vitamins ( A , D , E , K ) if patients are prone to such a deficiency anyway.
- Colestyramine can have a very negative effect on the absorption of thyroid hormones. When taking it, it is best to keep an interval of 4 hours between taking thyroid hormones and taking colestyramine. In addition, thyroxine and triiodothyronine are subject to the enterohepatic cycle. This means that the plasma level of thyroxine is reduced by colestyramine, which usually makes a (sometimes significant) increase in dose necessary. (Unfortunately, there are no clear sources of the exact effects of triiodothyronine.)
Side effects
Very often , the use of cholestyramine leads to constipation . Frequently also occur nausea , bloating , heartburn , indigestion , nausea, bloating and diarrhea on, occasionally nausea leads to vomiting . Increased fat stool , decreased absorption of fat-soluble vitamins ( A , D , E , K ) and decreased serum folic acid concentration were observed very rarely . In individual cases (without specifying the frequency) an increase in certain liver values (transaminases), a special metabolic disorder in patients with chronic kidney failure, as well as skin irritation, reddening of the skin and allergic reactions have been reported.
Trade names
Ipocol (CH), Lipocol (D), Quantalan (D, A, CH), Vasosan (D), various generics (D)
Individual evidence
- ↑ a b European Pharmacopoeia Commission (ed.): EUROPEAN PHARMACOPOE 5TH EDITION . tape 5.0 - 5.7 , 2006.
- ↑ a b Data sheet Cholestyramine resin from Sigma-Aldrich , accessed on December 27, 2012 ( PDF ).
- ↑ a b Entry on colestyramine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b c d e f g h i j k Specialist information Colestyramine, as of June 2005
- ↑ Red List Online, as of September 2009.
- ↑ AM comp. D. Switzerland, as of September 2009.
- ↑ AGES-PharmMed, as of September 2009.