Hydrochlorothiazide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
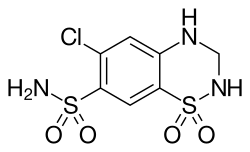
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Hydrochlorothiazide | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 7 H 8 ClN 3 O 4 S 2 | ||||||||||||||||||
| Brief description |
White to almost white, crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 297.74 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
274 ° C |
||||||||||||||||||
| pK s value |
7.9 |
||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Hydrochlorothiazide ( HCT or also called HTZ ) is a diuretic substance from the group of thiazide diuretics , the prototype of which it is considered to be. It is used for high blood pressure , heart failure or to flush out edema , often in the form of fixed combinations with other active ingredients (see commercial preparations ).
chemistry
The heterocyclic compound 6-chloro-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine-1,1-dioxide was discovered in the late 1950s by researchers at the US subsidiary of the Swiss Ciba in Summit (New Jersey) and described as a highly effective diuretic in Experientia magazine in 1958 . Manufacture has been protected by various patents, including a. U.S. Patent 3163645 (de Stevens, Werner, 1964). As the common name of the drug expresses, the compound can be regarded as the hydrogenated form of chlorothiazide , which was developed by Merck & Co. (USA) as a diuretic and antihypertensive agent. The preparation of hydrochlorothiazide and related compounds is described in U.S. Patent 3,025,292.
pharmacology
Mechanism of action
As a thiazide, hydrochlorothiazide reversibly inhibits the sodium - chloride cotransporter in the luminal cell membrane of the cells of the distal tubule in the kidney , whereby sodium chloride and the associated water of solution are excreted. Furthermore, the increases renal excretion of calcium - cations , and that of magnesium cations to. The increased calcium retention can lead to an increase in bone density in osteoporosis patients.
In high doses, hydrochlorothiazide also has an inhibitory effect on the enzyme carbonic anhydrase , but it has only a tenth of the inhibitory potency customary for carbonic anhydrase inhibitors and - like other thiazides - has a flat dose-effect curve , which means that only when the dose is increased enormously a minimal increase in further effectiveness is achieved. Hydrochlorothiazide should be avoided during pregnancy.
Pharmacokinetics
The bioavailability of hydrochlorothiazide is 70%, the plasma protein binding 95%, its duration of action 6–12 hours. Most of it (92 ± 5%) is excreted unchanged via the kidneys and has a plasma half-life of 1.3 to 1.7 hours.
Diuretics - u. a. Thiazide diuretics such as hydrochlorothiazide - through the loss of electrolytes and fluid, lead to an activation of the RAAS and thus to secondary hyperaldosteronism .
Analytics
For the reliable qualitative and quantitative determination of hydrochlorothiazide, the coupling of HPLC and mass spectrometry can be used after adequate sample preparation . The methodology is also used to detect adulteration of drugs.
Side effects
Description of side effects by frequency:
Common: disorders in the fluid and electrolyte balance (especially low potassium and sodium levels ( hypokalaemia and hyponatremia )), decreased magnesium and chloride levels, increased calcium levels in the blood ( hypomagnesaemia , hypochloremia , hypercalcemia ), dry mouth and thirst (with high doses, weakness - and dizziness, muscle pain and muscle cramps, e.g. calf cramps), headache, nervousness, palpitations, reduced blood pressure ( hypotension ), circulatory disorders with reduced blood pressure (when changing from lying down to standing: orthostatic regulation disorders); Excessive urine excretion can lead to thickening of the blood ( haemoconcentration ) as a result of "dehydration" ( dehydration ) and reduced circulating blood volume ( hypovolemia ) , as well as thrombosis and embolism as a result of the haemoconcentration - especially in elderly patients or in the presence of venous diseases . As a result of hypokalaemia, tiredness, drowsiness, muscle weakness, abnormal sensations in the limbs ( paresthesia ), paralysis ( paresis ), apathy , adynamia of the smooth muscles with constipation and excessive accumulation of gas in the gastrointestinal tract ( meteorism ) or cardiac arrhythmias may occur. Increased magnesium excretion in the urine (hypermagnesiuria) is common and only occasionally manifests itself as a magnesium deficiency in the blood (hypomagnesimia). Gout attacks (due to increased uric acid levels in the blood hyperuricemia ), increased blood sugar level ( hyperglycaemia ), increased excretion of sugar in the urine ( glucosuria ), increase in blood lipids ( cholesterol , triglycerides ).
Uncommon: increase in urinary substances ( creatinine , urea ), increased amylase levels in the blood (hyperamylasemia), inflammation of the pancreas ( pancreatitis ), loss of appetite and gastrointestinal complaints (e.g. nausea, vomiting, diarrhea , pain and cramps in the abdomen)
Rarely: allergic skin reactions (e.g .: itching, reddening of the skin, rashes due to exposure to light, small spots of bleeding in the skin and mucous membrane ( purpura ), very itchy wheals ( urticaria )), drug fever or jaundice ( jaundice ), acute kidney inflammation ( interstitial nephritis ) Inflammation of blood vessels ( vasculitis ), reduction in white blood cells ( leukopenia ), reduction in blood platelets ( thrombocytopenia ), anemia due to blood formation disorders in the bone marrow ( aplastic anemia ), erectile dysfunction, low-grade visual disturbances (e.g. blurred vision, color visual disturbances, yellow vision), impairment of Formation of tears, existing myopia may worsen.
In October 2018, the marketing authorization holders informed via Rote-Hand-Brief that pharmacoepidemiological studies had shown an increased risk of developing basal cell carcinoma (basal cell carcinoma , white skin cancer) or squamous cell carcinoma (spinal cell carcinoma ) after exposure to increasing cumulative doses of hydrochlorothiazide. The recommendation was made that patients treated with hydrochlorothiazide should be checked regularly for skin changes and that suspicious changes should be checked "if necessary including a histological examination of biopsies ". For patients who have already suffered from the skin diseases mentioned, “it may be necessary to carefully consider the use of HCT again”.
Use in doping
Hydrochlorothiazide is on the World Anti-Doping Agency's Prohibited List . Although it is not directly used to improve performance, it can be used to disguise such doping agents, so it is known as a masking agent .
Study situation
Hydrochlorothiazide is usually only used as a blood pressure lowering agent when no adequate blood pressure lowering can be achieved with another active ingredient alone and two active ingredients are necessary. The recommendation to additionally prescribe hydrochlorothiazide under this condition is based on the results of the MRFIT study, in which four different antihypertensive drugs were tested with precisely this question. In the end, three active ingredients performed equally well, but testing with the fourth - hydrochlorothiazide - was terminated prematurely because of increased cardiovascular mortality. Subsequent analyzes of the MRFIT study confirm this observation and may lead to it. a. due to increased cardiac arrhythmias. Nevertheless, in today's guidelines, the statements made by the authors with regard to the three successful active ingredients are also transferred to the fourth, failed one - i.e. hydrochlorothiazide. In terms of stroke prevention, however, HCT appears to have advantages.
Trade names
Monopreparations Disalunil (D), Esidrex (CH), Esidrix (D), numerous generic (D) combination products
- with Aliskiren : Rasilez HCT (D, A, CH)
- with amiloride : Comilorid (CH), Diursan (D), Ecodurex (CH), Escoretic (CH), Loradur (A), Moduretic (A, CH), Rhefluin (CH)
- with benazepril : Cibadrex (D, CH), numerous generics (D)
- with bisoprolol : Bilol comp. (CH), Concor plus (D, CH), Rivacor plus (A), Lodoz (CH), numerous generics (D)
- with candesartan : Atacand plus (D, A, CH), Blopress plus (D, A, CH), numerous generics (D)
- with captopril : ACE inhibitors (D), Adocomp (D), Capozide forte (A), Cardiagen (D), Jutacor comp. (D), Tensobon comp. (D), numerous generics (D)
- with cilazapril : Dynorm Plus (D), Inhibace Plus (CH)
- with enalapril : Co-Acepril (CH), Co-Reniten (CH), Coenytyrol (A), Corvo HCT (D), Elpradil HCT (CH), Renacor (D), Renitec plus (A, CH), numerous generics ( D)
- with eprosartan : Emestar plus (D), Teveten plus (D, A, CH)
- with fosinopril : Dynacil comp. (D)
- with irbesartan : CoAprovel (D, A, CH), Karvezide (D, A)
- with Lisinopril : Acecomp (A), Acercomp (D), Co-Lisinostad (A), Co-Lisinopril (A), Prinzide (CH), Zestoretic (CH), numerous generics (D)
- with Losartan : Cosaar Plus (CH), Fortzaar (A), Lanosar comp. (A), Lorzaar plus (D), Losathia (A), numerous generics (D)
- with metoprolol : Beloc-ZOK comp. (D), Seloken retard plus (A), numerous generics (D)
- with nebivolol : Hypoloc plus HCT, (A), Nomexor plus HCT (A)
- with olmesartan : Olmetec (D, A, CH), Votum plus (D, CH)
- with quinalapril : Accuzide (D, A, CH)
- with Ramipril : Delix plus (D), Lannapril plus (A), Triatec comp. (CH), Tritazide (A), Vesdil plus (D), numerous generics (D)
- with spironolactone : spironothiazide (D)
- with Telmisartan : Kinzalkomb (D, A, CH), Micardis plus (D, A, CH), Pritor plus (A)
- with Triamteren : Diuretic Verla (D), Diu Venostasin (D), Dytide H (D, A), Nephral (D), Tri-Thiazid (D), Turfa gamma (D)
- with valsartan : Co-Diovan (D, A, CH), Cordinate (D), Provas (D), numerous generics (D)
- with verapamil : Isoptin RR plus (D)
- with Zofenopril : Bifril plus (A)
as a double combination:
- with amiloride + timolol : Moducrin (D)
- with amlodipine + valsartan: Dafiro HCT (D), Exforge HCT (D, CH)
- with atenolol + amiloride: Kalten (CH)
- with propranolol + triamterene: Beta-Turfa gamma (D), Dociteren (D)
- with triamterene + verapamil: confit (A)
Web links
- Swiss Medicines Compendium: Hydrochlorothiazide Preparations
literature
- Ernst Mutschler: drug effects. Textbook of pharmacology and toxicology; with introductory chapters in anatomy, physiology and pathophysiology. 8th edition. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2001, ISBN 978-3-8047-1763-3 , pp. 585-586.
- Heinz Lüllmann, Klaus Mohr, Lutz Hein: Pocket Atlas of Pharmacology. 5th edition. Georg Thieme Verlag, Stuttgart 2004, ISBN 978-3-13-707705-3 , pp. 168-169.
Individual evidence
- ↑ a b European Pharmacopoeia Commission (Ed.): European Pharmacopoeia 5th Edition . tape 5.0-5.8 , 2006.
- ↑ a b c d e Entry on hydrochlorothiazide in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ a b Data sheet Hydrochlorothiazide from Sigma-Aldrich , accessed on June 16, 2011 ( PDF ).
- ↑ Jie Jack Lee: History of Drug Discovery . In: Jie Jack Li, EJ Corey (Eds.): Drug Discovery: Practices, Processes, and Perspectives . John Wiley & Sons, 2013, pp. 12 . ( limited preview in Google Book search)
- ↑ G. de Stevens, LH Werner, A. Halamandaris and S. Ricca Jr .: Dihydrobenzothiadiazine dioxides with potent diuretic effect . In: Experientia 14, 463 (1958), doi: 10.1007 / BF02327380 .
- ^ A b Eduard Burgis: Intensive course in general and special pharmacology. 3. Edition. Urban and Fischer (Elsevier), Munich 2004, ISBN 978-3-437-42613-1 , pp. 186-188.
- ↑ Comprehensive Heterocyclic Chemistry . 1 , pp. 173-174.
- ↑ Thomas Küttler: short textbook general pharmacology and toxicology. 18th edition. Urban and Fischer (Elsevier), Munich 2002, ISBN 978-3-437-41041-3 , p. 163.
- ↑ NV Ramakrishna, KN Vishwottam, S Manoj, M Koteshwara, S Wishu, DP Varma: Sensitive liquid chromatography-tandem mass spectrometry method for quantification of hydrochlorothiazide in human plasma . In: Biomed Chromatogr. , 2005 Dec, 19 (10), pp. 751-760, PMID 15856489
- ↑ JR Patel, TM Pethani, AN Vachhani, NR Sheth, AV Dudhrejiya: Development and validation of bioanalytical method for simultaneous estimation of ramipril and hydrochlorothiazide in human plasma using liquid chromatography-tandem mass spectrometry . In: J Chromatogr B Analyt Technol Biomed Life Sci. , 2014 Nov 1, 970, pp. 53-59, PMID 25240204
- ↑ M Bernard, W Akrout, CT Van Buu, C Metz, M Antignac, N Yagoubi, B Do: Liquid chromatography with tandem mass spectrometry for the simultaneous identification and quantification of cardiovascular drugs applied to the detection of substandard and falsified drugs . In: J Sep Sci. , 2015 Feb, 38 (4), pp. 562-570, PMID 25521603
- ↑ Instructions for use for HCT-beta from beta-pharm Arzneimittel GmbH, Augsburg (6/2008).
- ↑ media.gelbe-liste.de: Hydrochlorothiazide - Risk of non-melanoma skin cancer (PDF)
- ↑ The World Anti-Doping Code. The 2011 Prohibited List (international standard). (PDF; 125 kB) National Anti-Doping Agency, Bonn.
- ^ P Kezdi, PC Kezdi, HJ. Khamis: Diuretic induced long term hemodynamic changes in hypertension. A retrospective study in a MRFIT clinical center . In: Clin Exp Hypertens A , 1992, 14 (3), pp. 347-365.
- ↑ WE. Miall: Beta-blockers vs. thiazides in the treatment of hypertension: a review of the experience of the large national trials . In: J Cardiovasc Pharmacol , 1990, 16 Suppl 5, pp. 58-63.