Amlodipine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Racemate: structural formula without atropisomerism | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Amlodipine | |||||||||||||||||||||
| other names |
( RS ) -3-Ethyl-5-methyl-2 - [(2-aminoethoxy) methyl] -4- (2-chlorophenyl) -6-methyl-1,4-dihydro-3,5-pyridinedicarboxylate ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 408.88 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| solubility |
low in water (amlodipine hydrogen benzene sulfonate) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Amlodipine is a blood pressure lowering drug from the group of calcium channel blockers (calcium antagonists) of the nifedipine type. It is used as a basic therapeutic agent for essential high blood pressure ( arterial hypertension ). In the case of chronic stable angina pectoris and Prinzmetal's angina (vasospastic angina pectoris), it is used not only for treatment but also to prevent attacks.
In contrast to other active substances from this group, amlodipine has a comparatively long plasma half-life .
Mode of action
As a calcium channel antagonist of the nifedipine type ( 1,4-dihydropyridine ), amlodipine blocks the calcium channel of the L type by binding to the α 1 subunit. The reduced calcium influx into the smooth muscle cells reduces the muscle tone and thus primarily the vascular resistance and, in higher doses, the contractility (negative inotropic ) and the oxygen consumption in the heart muscle cells . In contrast to the calcium channel antagonists of the verapamil type, the dihydropyridines have a vascular selectivity, so that cardiac effects only occur at high doses that are not required for vasodilation (vasodilation). In the usual dosage, amlodipine has a dilating effect on the coronary vessels and the peripheral resistance vessels. Amlodipine also acts as a FIASMA (functional inhibitor of acid sphingomyelinase ).
Amlodipine is used pharmaceutically as a salt of benzenesulfonic acid (amlodipine besylate), methanesulfonic acid (amlodipine mesilate) or maleic acid (amlodipine maleate ). They are effective after oral administration.
Analytics
The reliable qualitative and quantitative determination of amlodipine and its metabolites in various test materials is possible after sufficient sample preparation by coupling high-performance liquid chromatography with mass spectrometry .
Pharmacokinetics
An important difference to other dihydropyridines (e.g. nifedipine ) is the long terminal half-life of around 35 to 50 hours, which enables once-a-day administration and prevents cardiovascular complications caused by fluctuations in blood pressure.
Side effects
The side effects correspond to those of the active ingredient group of calcium antagonists . A typical side effect of dihydropyridines can be edema of the legs and, especially at the beginning of treatment, headaches and reddening of the face with sensation of heat. Dizziness , tiredness, palpitations of the heart, abdominal pain and nausea are also common, and vomiting may occasionally occur. In very rare cases, allergic reactions can occur. Another side effect: gum growths (often bullous , but mostly free of inflammation). These usually disappear again after a dose reduction or a change of medication.
Contraindications
Treatment with amlodipine is not indicated in cases of hypersensitivity to the substance or other dihydropyridine derivatives, in the case of cardiovascular shock, severe aortic stenosis , unstable angina pectoris or severe liver dysfunction.
Trade names
- Monopreparations : Norvasc (A, D), various generics (D, A, CH)
-
Combination preparations :
with valsartan : Copalia (A), Dafiro (A, D), Exforge (A, D, CH), Imprida (A)
with atorvastatin : Caduet (A, CH)
with olmesartan : Amelior (A), Vascord (CH ) Vocado (D), Sevikar (A, C, D)
with ramipril : Tonotec (D)
with telmisartan : Twynsta (EU)
with bisoprolol : Bisodipin (D)
with Lisinopril : Lisam (A)
with candesartan : Camlostar (D) - Veterinary medicine: Amodip
synthesis
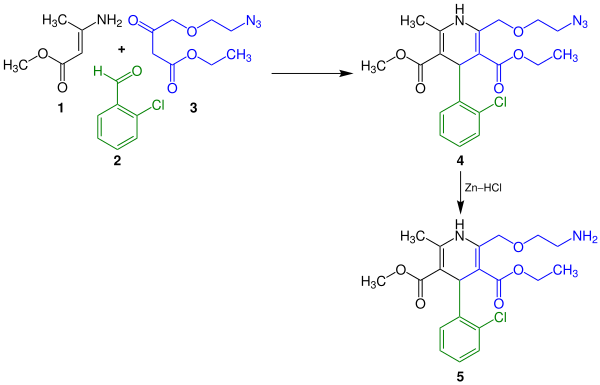
Amlodipine can be made in a two-step reaction. For this purpose, the carboxylic acid esters 1 and 3 react with 2-chlorobenzaldehyde ( 2 ) in a multi-component reaction . The pyridine derivative 4 is formed in this condensation reaction , which then forms the drug amlodipine ( 5 ) with the addition of zinc and hydrogen chloride with a palladium catalyst :
Stereoisomerism
Amlodipine is marketed as a racemate [1: 1 mixture of ( R ) - (+) - and ( S ) - (-) - amlodipine]. A method for the semi-preparative chromatographic separation of the enantiomers ( S ) - (-) - amlodipine and ( R ) - (+) - amlodipine is known. The table shows both stereoisomers, more precisely atropisomers . They differ in the position of the hydrogen atom, which is either above or below the plane of the ring.
| Amlodipine stereoisomers | |
| ( R ) -enantiomer | ( S ) -enantiomer |

|

|
Individual evidence
- ↑ a b Amlodipine data sheet at AlfaAesar, accessed on January 14, 2020 ( PDF )(JavaScript required) .
- ↑ a b The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. 2006, ISBN 0-911910-00-X , p. 83.
- ↑ Entry on 3-ethyl-5-methyl-2- (2-aminoethoxymethyl) -4- (2-chlorophenyl) -1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Entry on 3-ethyl-5-methyl-2- (2-aminoethoxymethyl) -4- (2-chlorophenyl) -1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on March 15, 2017. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ soot; Endres: Medicines pocket plus 2008. 4th edition. 2007, ISBN 978-3-89862-287-5 .
- ↑ J. Kornhuber, M. Muehlbacher, S. Trapp, S. Pechmann, A. Friedl, M. Reichel, C. Mühle, L. Terfloth, T. Groemer, G. Spitzer, K. Liedl, E. Gulbins, P Tripal: Identification of Novel Functional Inhibitors of Acid Sphingomyelinase . In: PLoS ONE . tape 6 , no. 8 , 2011, p. e23852 , doi : 10.1371 / journal.pone.0023852 .
- ^ JO Johannsen, H. Reuter, F. Hoffmann, C. Blaich, MHJ Wiesen, T. Streichert, C. Müller: Reliable and easy-to-use LC-MS / MS-method for simultaneous determination of the antihypertensives metoprolol, amlodipine , canrenone and hydrochlorothiazide in patients with therapy-refractory arterial hypertension. In: J Pharm Biomed Anal. 164, Nov 5, 2018, pp. 373-381. PMID 30439665
- ↑ L. Wang, W. Liu, Z. Zhang, Y. Tian: Validated LC-MS / MS method for the determination of amlodipine enantiomers in rat plasma and its application to a stereoselective pharmacokinetic study. In: J Pharm Biomed Anal. 158, Sep 5, 2018, pp. 74–81. PMID 29860181
- ↑ JV Shah, JM Parekh, PA Shah, PV Shah, M. Sanyal, PS Shrivastav: Application of an LC-MS / MS method for the analysis of amlodipine, valsartan and hydrochlorothiazide in polypill for a bioequivalence study. In: J Pharm Anal. 7 (5), Oct 2017, pp. 309-316. PMID 29404054
- ↑ JJ van der Hooft, S. Padmanabhan, KE Burgess, MP Barrett: Urinary antihypertensive drug metabolite screening using molecular networking coupled to high-resolution mass spectrometry fragmentation. In: Metabolomics. 12, 2016, p. 125. PMID 27471437
- ↑ Ernst Mutschler: Mutschler drug effects. Pharmacology, clinical pharmacology, toxicology. 10th edition. Stuttgart 2013, p. 507.
- ↑ technical information Norvasc October 2011th
- ↑ ABDA database (as of June 8, 2008) of DIMDI .
- ↑ Axel Kleemann , Jürgen Engel, Bernhard Kutscher, Dietmar Reichert: Pharmaceutical Substances - Syntheses, Patents and Applications of the most relevant APIs. 5th edition. Georg Thieme Verlag, 2009, ISBN 978-3-13-558405-8 , p. 66.
- ↑ J. Lukša, D. Josič, M. Kremser, Z. Kopitar, S. Milutinovič: Pharmacokinetic behavior of ( R ) - (+) - and ( S ) - (-) - amlodipine after single enantiomer administration. In: Journal of Chromatography B: Biomedical Sciences and Applications . 703, 1997, pp. 185-193. PMID 9448075 . doi: 10.1016 / S0378-4347 (97) 00394-0 .
- ↑ J. Lukša, D. Josič, B. Furlan, M. Kremser: Semi-preparative chromatographic purification of the enantiomers ( S ) - (-) - amlodipine and ( R ) - (+) - amlodipine. In: Journal of Chromatography B: Biomedical Sciences and Applications. 693, 1997, pp. 367-375. doi: 10.1016 / S0378-4347 (97) 00069-8 .