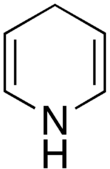
1,4-dihydropyridine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | 1,4-dihydropyridine | |||||||||
| other names |
Dihydropyridine |
|||||||||
| Molecular formula | C 5 H 7 N | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 81.12 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Dihydropyridine is a chemical compound consisting of a six-membered unsaturated ring with one nitrogen atom. It forms the basic structure of the dihydropyridines , which are used in medicine as calcium channel blockers in arterial hypertension . Its unsaturated analogue is pyridine .
presentation
The reduction of pyridine using lithium aluminum hydride gives a mixture of 1,4-dihydropyridine, 1,2-dihydropyridine and 2,5-dihydropyridine . Pure 1,4-dihydropyridine is formed from pyridine in the presence of organic magnesium and zinc complexes.
The dihydropyridine synthesis according to Arthur Hantzsch is used to prepare more highly substituted pyridines .
Biological importance
Dihydropyridine derivatives (e.g. the lead substance nifedipine , amlodipine , isradipine , nitrendipine , nimodipine or felodipine ) can block the voltage-dependent L-type calcium channel , i.e. act as antagonists. Before the exact function of this channel was explained, it was therefore called the dihydropyridine receptor .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ DD Tanner, Yang, C.-M .: On the structure and mechanism of formation of the Lansbury reagent, lithium tetrakis (N-dihydropyridyl) aluminate , in J. Org. Chem. , 1993 , 58 , 1840-1846 doi : 10.1021 / jo00059a041 .
- ^ DM Stout, AI Meyers: Recent advances in the chemistry of dihydropyridines , in Chem. Rev. , 1982 , 82 , 223-243 doi : 10.1021 / cr00048a004 .
- ↑ AJ De Koning, PHM Budzelaar, J. Boersma, GJM van der Kerk: Specific and selective reduction of aromatic nitrogen heterocycles with the bis-pyridine complexes of bis (1,4-dihydro-1-pyridyl) zinc and bis (1, 4-dihydro-1-pyridyl) magnesium , in J. Organomet. Chem. , 1980 , 199 , 153-170 doi : 10.1016 / S0022-328X (00) 83849-8 .
- ↑ Joachim Buddrus: Fundamentals of organic chemistry . 3rd edition, 2003, p. 360.