Telmisartan
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
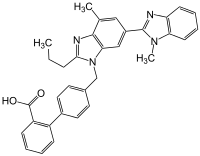
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Telmisartan | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 33 H 30 N 4 O 2 | |||||||||||||||||||||
| Brief description |
white solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 514.63 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
261-263 ° C |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Telmisartan is a drug from the group of selective AT 1 antagonists ("sartans") and is used to treat essential high blood pressure .
Clinical indications and pharmacology
As with many sartans, the side effects are at the placebo level. Rarely can Somnolenzen , hyperkalemia , constipation and a creatinine -increase come. Administration is contraindicated in patients with severe liver insufficiency and in the second and last trimester of pregnancy. There are precautionary measures and warnings for the first trimester of pregnancy and for patients wishing to have children, use with simultaneous breastfeeding , as well as with impaired kidney function and mild and moderate liver dysfunction.
The bioavailability of telmisartan is around 40% and the half-life is 24 hours. Telmisartan is almost completely (over 99.5%) bound to plasma proteins. Excretion occurs almost exclusively in the stool ( faeces ).
Telmisartan has been approved in the EU since 2013 for the treatment of proteinuria in chronic kidney disease in cats under the brand name Semintra .
chemistry
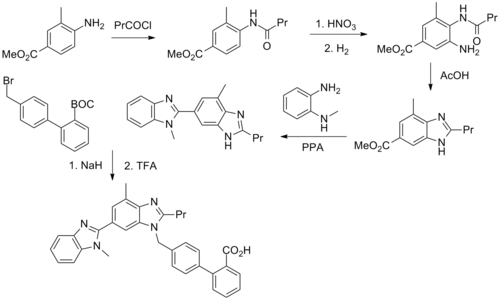
Telmisartan is a derivative of biphenyl-2-carboxylic acid and contains two benzimidazole groups. The multistep synthesis of telmisartan - starting from 4-amino-3-methyl-benzoic acid methyl ester - is described in the literature:
"Pr" stands for an n -propyl group.
Trade names
Monopreparations : Micardis (EU, CH, USA), Kinzalmono (EU), Tolura
Veterinary medicine: Semintra (EU)
- with hydrochlorothiazide : MicardisPlus (EU, CH), Micardis HCT (USA), Kinzalkomb (EU)
- with amlodipine : Twynsta (EU, USA)
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Telmisartan
Individual evidence
- ↑ a b Datasheet Telmisartan, 99% from AlfaAesar, accessed on April 28, 2014 ( PDF )(JavaScript required) .
- ↑ a b c d Datasheet Telmisartan, ≥98% (HPLC), solid from Sigma-Aldrich , accessed on April 28, 2014 ( PDF ).
- ↑ Takács-Novák, K .; Urac, M .; Horváth, P .; Völgyi, G .; Anderson, BD; Avdeef, A .: Equilibrium solubility measurement of compounds with low dissolution rate by Higuchi's Facilitated Dissolution Method. A validation study in Eur. J. Pharm. Sci. 106 (2017) 133-144, doi : 10.1016 / j.ejps.2017.05.064 .
- ↑ a b Technical information Micardis , as of March 2013.
- ↑ Ernst Mutschler, Monika Schäfer-Korting: Textbook of Pharmacology and Toxicology . 8th edition, Wissenschaftliche Verlagsgesellschaft Stuttgart 2001. ISBN 3-8047-1763-2 , p. 581.
- ^ Committee for Medicinal Products for Veterinary Use: CVMP assessment report for Semintra (EMEA / V / C / 002436) of December 13, 2012.
- ↑ New treatment for cats with chronic kidney disease: Boehringer Ingelheim launches Semintra®. ( Memento of the original from May 22, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Press release from Boehringer Ingelheim dated September 2, 2013.
- ↑ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dieter Reichert: Pharmaceutical Substances , 4th edition (2000) 2 volumes published by Thieme-Verlag Stuttgart, pp. 1978–1979, ISBN 978-1-58890-031-9 ; online since 2003 with biannual additions and updates.
- ↑ ROTE LISTE 2013 , Verlag Rote Liste Service GmbH, Frankfurt am Main, ISBN 978-3-939192-70-1 .